Tissue Localization and Variation of Major Symbionts in Haemaphysalis longicornis, Rhipicephalus haemaphysaloides, and Dermacentor silvarum in China
- PMID: 29523550
- PMCID: PMC5930374
- DOI: 10.1128/AEM.00029-18
Tissue Localization and Variation of Major Symbionts in Haemaphysalis longicornis, Rhipicephalus haemaphysaloides, and Dermacentor silvarum in China
Abstract
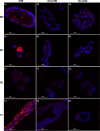
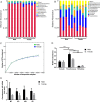
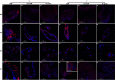
Ticks are important disease vectors, as they transmit a variety of human and animal pathogens worldwide. Symbionts that coevolved with ticks confer crucial benefits to their host in nutrition metabolism, fecundity, and vector competence. Although over 100 tick species have been identified in China, general information on tick symbiosis is limited. Here, we visualized the tissue distribution of Coxiella sp. and Rickettsia sp. in lab-reared Haemaphysalis longicornis and Rhipicephalus haemaphysaloides by fluorescent in situ hybridization. We found that Coxiella sp. colonized exclusively the Malpighian tubules and ovaries of H. longicornis, while Rickettsia sp. additionally colonized the midgut of R. haemaphysaloides We also investigated the population structure of microbiota in Dermacentor silvarum ticks collected from Inner Mongolia, China, and found that Coxiella, Rickettsia, and Pseudomonas are the three dominant genera. No significant difference in microbiota composition was found between male and female D. silvarum ticks. We again analyzed the tissue localization of Coxiella sp. and Rickettsia sp. and found that they displayed tissue tropisms similar to those in R. haemaphysaloides, except that Rickettsia sp. colonized the nuclei of spermatids instead of ovaries in D. silvarum Altogether, our results suggest that Coxiella sp. and Rickettsia sp. are the main symbionts in the three ticks and reside primarily in midgut, Malpighian tubules, and reproductive tissues, but their tissue distribution varies in association with species and sexes.IMPORTANCE Tick-borne diseases constitute a major public health burden, as they are increasing in frequency and severity worldwide. The presence of symbionts helps ticks to metabolize nutrients, promotes fecundity, and influences pathogen infections. Increasing numbers of tick-borne pathogens have been identified in China; however, knowledge of native ticks, especially tick symbiosis, is limited. In this study, we analyze the distribution of Coxiella sp. and Rickettsia sp. in tissues of laboratory-reared Haemaphysalis longicornis and Rhipicephalus haemaphysaloides and field-collected Dermacentor silvarum We found that the localization patterns of Coxiella sp. in three Chinese tick species were similar to those of other tick species. We also found a previously undefined intracellular localization of Rickettsia sp. in tick midgut and spermatids. In addition, we demonstrate that tissue tropisms of symbionts vary between species and sexes. Our findings provide new insights into the tissue localization of symbionts in native Chinese ticks and pave the way for further understanding of their functional capabilities and symbiotic interactions with ticks.
Keywords: Coxiella; Rickettsia; microbiota; ticks; tissue localization.
Copyright © 2018 American Society for Microbiology.
Figures




References
-
- WHO. 2017. Global vector control response 2017–2030. World Health Organization, Geneva, Switzerland.
-
- Fang LQ, Liu K, Li XL, Liang S, Yang Y, Yao HW, Sun RX, Sun Y, Chen WJ, Zuo SQ, Ma MJ, Li H, Jiang JF, Liu W, Yang XF, Gray GC, Krause PJ, Cao WC. 2015. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis 15:1467–1479. doi: 10.1016/S1473-3099(15)00177-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

