Lysosomal Cholesterol Hydrolysis Couples Efferocytosis to Anti-Inflammatory Oxysterol Production
- PMID: 29523554
- PMCID: PMC6034181
- DOI: 10.1161/CIRCRESAHA.117.312333
Lysosomal Cholesterol Hydrolysis Couples Efferocytosis to Anti-Inflammatory Oxysterol Production
Abstract
Rationale: Macrophages face a substantial amount of cholesterol after the ingestion of apoptotic cells, and the LIPA (lysosomal acid lipase) has a major role in hydrolyzing cholesteryl esters in the endocytic compartment.
Objective: Here, we directly investigated the role of LIPA-mediated clearance of apoptotic cells both in vitro and in vivo.
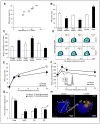
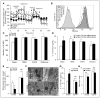
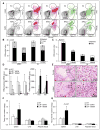
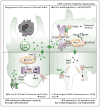
Methods and results: We show that LIPA inhibition causes a defective efferocytic response because of impaired generation of 25-hydroxycholesterol and 27-hydroxycholesterol. Reduced synthesis of 25-hydroxycholesterol after LIPA inhibition contributed to defective mitochondria-associated membrane leading to mitochondrial oxidative stress-induced NLRP3 (NOD-like receptor family, pyrin domain containing) inflammasome activation and caspase-1-dependent Rac1 (Ras-related C3 botulinum toxin substrate 1) degradation. A secondary event consisting of failure to appropriately activate liver X receptor-mediated pathways led to mitigation of cholesterol efflux and apoptotic cell clearance. In mice, LIPA inhibition caused defective clearance of apoptotic lymphocytes and stressed erythrocytes by hepatic and splenic macrophages, culminating in splenomegaly and splenic iron accumulation under hypercholesterolemia.
Conclusions: Our findings position lysosomal cholesterol hydrolysis as a critical process that prevents metabolic inflammation by enabling efficient macrophage apoptotic cell clearance.
Keywords: cholesterol; inflammation; macrophage; mitochondria; oxysterols.
© 2018 American Heart Association, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

