Patterns and trends of potentially inappropriate high-density lipoprotein cholesterol testing in Australian adults at high risk of cardiovascular disease from 2008 to 2014: analysis of linked individual patient data from the Australian Medicare Benefits Schedule and Pharmaceutical Benefits Scheme
- PMID: 29523561
- PMCID: PMC5855213
- DOI: 10.1136/bmjopen-2017-019041
Patterns and trends of potentially inappropriate high-density lipoprotein cholesterol testing in Australian adults at high risk of cardiovascular disease from 2008 to 2014: analysis of linked individual patient data from the Australian Medicare Benefits Schedule and Pharmaceutical Benefits Scheme
Abstract
Objectives: We examine the extent to which the adult Australian population on lipid-lowering medications receives the level of high-density lipoprotein cholesterol (HDL-C) testing recommended by national guidelines.
Data: We analysed records from 7 years (2008-2014) of the 10% publicly available sample of deidentified, individual level, linked Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS) electronic databases of Australia.
Methods: The PBS data were used to identify individuals on stable prescriptions of lipid-lowering treatment. The MBS data were used to estimate the annual frequency of HDL-C testing. We developed a methodology to address the issue of 'episode coning' in the MBS data, which causes an undercounting of pathology tests. We used a published figure on the proportion of unreported HDL-C tests to correct for the undercounting and estimate the probability that an HDL-C test was performed. We judged appropriateness of testing frequency by comparing the HDL-C testing rate to guidelines' recommendations of annual testing for people at high risk for cardiovascular disease.
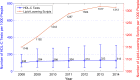
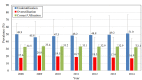
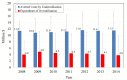
Results: We estimated that approximately 49% of the population on stable lipid-lowering treatment did not receive any HDL-C test in a given year. We also found that approximately 19% of the same population received two or more HDL-C tests within the year. These levels of underutilisation and overutilisation have been changing at an average rate of 2% and -4% a year, respectively, since 2009. The yearly expenditure associated with test overutilisation was approximately $A4.3 million during the study period, while the cost averted because of test underutilisation was approximately $A11.3 million a year.
Conclusions: We found that approximately half of Australians on stable lipid-lowering treatment may be having fewer HDL-C testing than recommended by national guidelines, while nearly one-fifth are having more tests than recommended.
Keywords: cardiac epidemiology; chemical pathology; epidemiology; primary care.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Non-high-density lipoprotein cholesterol, guideline targets, and population percentiles for secondary prevention in 1.3 million adults: the VLDL-2 study (very large database of lipids).J Am Coll Cardiol. 2013 Nov 19;62(21):1960-1965. doi: 10.1016/j.jacc.2013.07.045. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973689 Clinical Trial.
-
Cholesterol-lowering therapy and the Australian Pharmaceutical Benefits Scheme: a population study.Aust Health Rev. 2009 May;33(2):325-33. doi: 10.1071/ah090325. Aust Health Rev. 2009. PMID: 19563324
-
How do the Australian guidelines for lipid-lowering drugs perform in practice? Cardiovascular disease risk in the AusDiab Study, 1999-2000.Med J Aust. 2008 Sep 15;189(6):319-22. doi: 10.5694/j.1326-5377.2008.tb02049.x. Med J Aust. 2008. PMID: 18803535
-
Medical lipid-regulating therapy: current evidence, ongoing trials and future developments.Drugs. 2004;64(11):1181-96. doi: 10.2165/00003495-200464110-00003. Drugs. 2004. PMID: 15161326 Review.
-
'Trig-onometry': non-high-density lipoprotein cholesterol as a therapeutic target in dyslipidaemia.Int J Clin Pract. 2011 Jan;65(1):82-101. doi: 10.1111/j.1742-1241.2010.02547.x. Epub 2010 Nov 24. Int J Clin Pract. 2011. PMID: 21105969 Review.
Cited by
-
Predicting diabetes second-line therapy initiation in the Australian population via time span-guided neural attention network.PLoS One. 2019 Oct 18;14(10):e0211844. doi: 10.1371/journal.pone.0211844. eCollection 2019. PLoS One. 2019. PMID: 31626666 Free PMC article.
-
What methods are being used to create an evidence base on the use of laboratory tests to monitor long-term conditions in primary care? A scoping review.Fam Pract. 2020 Nov 28;37(6):845-853. doi: 10.1093/fampra/cmaa074. Fam Pract. 2020. PMID: 32820328 Free PMC article.
-
Real-World Research on Retinal Diseases Using Health Claims Database: A Narrative Review.Diagnostics (Basel). 2024 Jul 19;14(14):1568. doi: 10.3390/diagnostics14141568. Diagnostics (Basel). 2024. PMID: 39061705 Free PMC article. Review.
References
-
- GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1603–58. 10.1016/S0140-6736(16)31460-X - DOI - PMC - PubMed
-
- Australian Institute of Health and Welfare. Australian burden of disease study: impact and causes of illness and death in Australia 2011, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
