15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-κB Regulators in Bacterial Pneumonia
- PMID: 29523657
- PMCID: PMC5906795
- DOI: 10.4049/jimmunol.1602090
15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-κB Regulators in Bacterial Pneumonia
Abstract
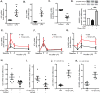
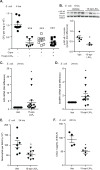
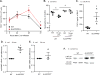
Specialized proresolving mediators (SPMs) decrease NF-κB activity to prevent excessive tissue damage and promote the resolution of acute inflammation. Mechanisms for NF-κB regulation by SPMs remain to be determined. In this study, after LPS challenge, the SPMs 15-epi-lipoxin A4 (15-epi-LXA4), resolvin D1, resolvin D2, resolvin D3, and 17-epi-resolvin D1 were produced in vivo in murine lungs. In LPS-activated human bronchial epithelial cells, select SPMs increased expression of the NF-κB regulators A20 and single Ig IL-1R-related molecule (SIGIRR). Of interest, 15-epi-LXA4 induced A20 and SIGIRR in an lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2) receptor-dependent manner in epithelial cells and in murine pneumonia. This SPM regulated NF-κB-induced cytokines to decrease pathogen-mediated inflammation. In addition to dampening lung inflammation, surprisingly, 15-epi-LXA4 also enhanced pathogen clearance with increased antimicrobial peptide expression. Taken together, to our knowledge these results are the first to identify endogenous agonists for A20 and SIGIRR expression to regulate NF-κB activity and to establish mechanisms for NF-κB regulation by SPMs for pneumonia resolution.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
C.N.S. is an inventor on patents (resolvins) assigned to BWH and licensed for clinical development. The interests of C.N.S. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. B.D.L. is an inventor on patents (resolvins) assigned to BWH and licensed to Resolvyx Pharmaceuticals. The interests of B.D.L. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. The remaining authors declare no conflict of interest.
Figures

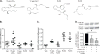

Similar articles
-
Resolvin D1 inhibits TGF-β1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32.Int J Biochem Cell Biol. 2013 Dec;45(12):2801-7. doi: 10.1016/j.biocel.2013.09.018. Epub 2013 Oct 10. Int J Biochem Cell Biol. 2013. PMID: 24120851
-
Lipoxin Signaling in Murine Lung Host Responses to Cryptococcus neoformans Infection.Am J Respir Cell Mol Biol. 2016 Jan;54(1):25-33. doi: 10.1165/rcmb.2014-0102OC. Am J Respir Cell Mol Biol. 2016. PMID: 26039320 Free PMC article.
-
Inhibition of the lipoxin A4 and resolvin D1 receptor impairs host response to acute lung injury caused by pneumococcal pneumonia in mice.Am J Physiol Lung Cell Mol Physiol. 2021 Jun 1;320(6):L1085-L1092. doi: 10.1152/ajplung.00046.2021. Epub 2021 Apr 6. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33822656 Free PMC article.
-
Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation.Inflamm Allergy Drug Targets. 2006 Apr;5(2):91-106. doi: 10.2174/187152806776383125. Inflamm Allergy Drug Targets. 2006. PMID: 16613568 Review.
-
Specialized pro-resolving mediator network: an update on production and actions.Essays Biochem. 2020 Sep 23;64(3):443-462. doi: 10.1042/EBC20200018. Essays Biochem. 2020. PMID: 32885825 Free PMC article. Review.
Cited by
-
Hypoxia-inducible factor-dependent induction of myeloid-derived netrin-1 attenuates natural killer cell infiltration during endotoxin-induced lung injury.FASEB J. 2021 Apr;35(4):e21334. doi: 10.1096/fj.202002407R. FASEB J. 2021. PMID: 33715200 Free PMC article.
-
Specialized Pro-resolving Mediators Reduce Pro-nociceptive Inflammatory Mediator Production in Models of Localized Provoked Vulvodynia.J Pain. 2021 Oct;22(10):1195-1209. doi: 10.1016/j.jpain.2021.03.144. Epub 2021 Apr 1. J Pain. 2021. PMID: 33813057 Free PMC article.
-
Sepsis therapies: learning from 30 years of failure of translational research to propose new leads.EMBO Mol Med. 2020 Apr 7;12(4):e10128. doi: 10.15252/emmm.201810128. Epub 2020 Mar 16. EMBO Mol Med. 2020. PMID: 32176432 Free PMC article. Review.
-
Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections.Nutrients. 2020 Apr 23;12(4):1181. doi: 10.3390/nu12041181. Nutrients. 2020. PMID: 32340216 Free PMC article. Review.
-
Pro-Resolving Ligands Orchestrate Phagocytosis.Front Immunol. 2021 Jun 10;12:660865. doi: 10.3389/fimmu.2021.660865. eCollection 2021. Front Immunol. 2021. PMID: 34177900 Free PMC article. Review.
References
-
- Gilroy D, De Maeyer R. New insights into the resolution of inflammation. Semin Immunol. 2015;27:161–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

