The length of the interleukin-11 receptor stalk determines its capacity for classic signaling
- PMID: 29523682
- PMCID: PMC5925790
- DOI: 10.1074/jbc.RA118.001879
The length of the interleukin-11 receptor stalk determines its capacity for classic signaling
Abstract
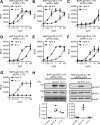
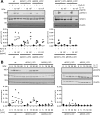
Interleukin (IL)-11 is a multifunctional cytokine that was traditionally recognized for its hematopoietic and anti-inflammatory functions, but has recently been shown also to be involved in tumorigenesis. IL-11 signaling is initiated by binding of the cytokine to the IL-11 receptor (IL-11R), which is not directly involved in signaling but required for IL-11 binding to the signal-transducing receptor glycoprotein (gp) 130. In classic signaling, IL-11 binds to the membrane-bound IL-11R to initiate signal transduction. Additionally, IL-11 signaling can be initiated via soluble IL-11R, known as trans-signaling, and this pathway only requires the three extracellular domains of the IL-11R, but not stalk, transmembrane, or intracellular region. Here, we analyzed the role of the IL-11R stalk region, a 55 amino acid stretch connecting the extracellular domains with the transmembrane helix, in classic IL-11 signaling with the help of cytokine-dependent cell lines. We showed that the stalk region is crucial for IL-11 signaling via the membrane-bound IL-11R. Using different deletion variants, we found that a minimal length of 23 amino acid residues is required for efficient signal transduction. We further found that classic IL-11 signaling depended solely on the length, but not the sequence, of the IL-11R stalk region, suggesting that the stalk functions as a spacer in the signaling complex. We previously described the IL-11R stalk region as determinant of proteolysis and regulator of IL-11 trans-signaling. The results presented here reveal an additional function in classic IL-11 signaling, highlighting the importance of the IL-11R stalk in IL-11 signaling.
Keywords: STAT3; cytokine; gp130; interleukin; interleukin-11; interleukin-11 receptor; proliferation; signal transduction; stalk region.
© 2018 Lokau and Garbers.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Paul S. R., Bennett F., Calvetti J. A., Kelleher K., Wood C. R., O'Hara R. M. Jr., Leary A. C., Sibley B., Clark S. C., and Williams D. A. (1990) Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc. Natl. Acad. Sci. U.S.A. 87, 7512–7516 10.1073/pnas.87.19.7512 - DOI - PMC - PubMed
-
- Orazi A., Cooper R. J., Tong J., Gordon M. S., Battiato L., Sledge G. W. Jr., Kaye J. A., Kahsai M., and Hoffman R. (1996) Effects of recombinant human interleukin-11 (Neumega rhIL-11 growth factor) on megakaryocytopoiesis in human bone marrow. Exp. Hematol. 24, 1289–1297 - PubMed
-
- Trepicchio W. L., Wang L., Bozza M., and Dorner A. J. (1997) IL-11 regulates macrophage effector function through the inhibition of nuclear factor-kappaB. J. Immunol. 159, 5661–5670 - PubMed
-
- Ernst M., Najdovska M., Grail D., Lundgren-May T., Buchert M., Tye H., Matthews V. B., Armes J., Bhathal P. S., Hughes N. R., Marcusson E. G., Karras J. G., Na S., Sedgwick J. D., Hertzog P. J., and Jenkins B. J. (2008) STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J. Clin. Invest. 118, 1727–1738 10.1172/JCI34944 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

