Purkinje cell BKchannel ablation induces abnormal rhythm in deep cerebellar nuclei and prevents LTD
- PMID: 29523816
- PMCID: PMC5845018
- DOI: 10.1038/s41598-018-22654-6
Purkinje cell BKchannel ablation induces abnormal rhythm in deep cerebellar nuclei and prevents LTD
Abstract
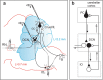
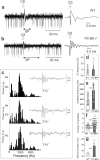
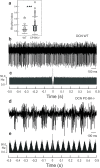
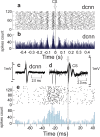
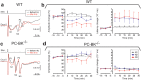
Purkinje cells (PC) control deep cerebellar nuclei (DCN), which in turn inhibit inferior olive nucleus, closing a positive feedback loop via climbing fibers. PC highly express potassium BK channels but their contribution to the olivo-cerebellar loop is not clear. Using multiple-unit recordings in alert mice we found in that selective deletion of BK channels in PC induces a decrease in their simple spike firing with a beta-range bursting pattern and fast intraburst frequency (~200 Hz). To determine the impact of this abnormal rhythm on the olivo-cerebellar loop we analyzed simultaneous rhythmicity in different cerebellar structures. We found that this abnormal PC rhythmicity is transmitted to DCN neurons with no effect on their mean firing frequency. Long term depression at the parallel-PC synapses was altered and the intra-burst complex spike spikelets frequency was increased without modification of the mean complex spike frequency in BK-PC-/- mice. We argue that the ataxia present in these conditional knockout mice could be explained by rhythmic disruptions transmitted from mutant PC to DCN but not by rate code modification only. This suggests a neuronal mechanism for ataxia with possible implications for human disease.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels.Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12323-8. doi: 10.1073/pnas.1001745107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566869 Free PMC article.
-
Unusually Slow Spike Frequency Adaptation in Deep Cerebellar Nuclei Neurons Preserves Linear Transformations on the Subsecond Timescale.J Neurosci. 2022 Oct 5;42(40):7581-7593. doi: 10.1523/JNEUROSCI.1869-21.2022. Epub 2022 Aug 22. J Neurosci. 2022. PMID: 35995561 Free PMC article.
-
BK and Kv3.1 potassium channels control different aspects of deep cerebellar nuclear neurons action potentials and spiking activity.Cerebellum. 2011 Dec;10(4):647-58. doi: 10.1007/s12311-011-0279-9. Cerebellum. 2011. PMID: 21750937
-
Increased Purkinje Cell Complex Spike and Deep Cerebellar Nucleus Synchrony as a Potential Basis for Syndromic Essential Tremor. A Review and Synthesis of the Literature.Cerebellum. 2021 Apr;20(2):266-281. doi: 10.1007/s12311-020-01197-5. Epub 2020 Oct 13. Cerebellum. 2021. PMID: 33048308 Review.
-
Time window control: a model for cerebellar function based on synchronization, reverberation, and time slicing.Prog Brain Res. 2000;124:275-97. doi: 10.1016/S0079-6123(00)24023-5. Prog Brain Res. 2000. PMID: 10943132 Review.
Cited by
-
BK Channel Regulation of Afterpotentials and Burst Firing in Cerebellar Purkinje Neurons.J Neurosci. 2021 Mar 31;41(13):2854-2869. doi: 10.1523/JNEUROSCI.0192-20.2021. Epub 2021 Feb 16. J Neurosci. 2021. PMID: 33593855 Free PMC article.
-
Cerebellar Cortex 4-12 Hz Oscillations and Unit Phase Relation in the Awake Rat.Front Syst Neurosci. 2020 Nov 10;14:475948. doi: 10.3389/fnsys.2020.475948. eCollection 2020. Front Syst Neurosci. 2020. PMID: 33240052 Free PMC article.
-
LINGO1 is a regulatory subunit of large conductance, Ca2+-activated potassium channels.Proc Natl Acad Sci U S A. 2020 Jan 28;117(4):2194-2200. doi: 10.1073/pnas.1916715117. Epub 2020 Jan 13. Proc Natl Acad Sci U S A. 2020. PMID: 31932443 Free PMC article.
-
Acute Cerebellar Inflammation and Related Ataxia: Mechanisms and Pathophysiology.Brain Sci. 2022 Mar 10;12(3):367. doi: 10.3390/brainsci12030367. Brain Sci. 2022. PMID: 35326323 Free PMC article. Review.
-
Targeting Ion Channels and Purkinje Neuron Intrinsic Membrane Excitability as a Therapeutic Strategy for Cerebellar Ataxia.Life (Basel). 2023 Jun 8;13(6):1350. doi: 10.3390/life13061350. Life (Basel). 2023. PMID: 37374132 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

