Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids
- PMID: 29524537
- PMCID: PMC6128784
- DOI: 10.1016/j.jaci.2017.12.1009
Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids
Abstract
Background: Airway type 2 inflammation is usually corticosteroid sensitive, but the role of type 2 inflammation as a mechanism of asthma in patients receiving high-dose inhaled corticosteroids (ICSs) is uncertain.
Objective: We sought to determine whether airway type 2 inflammation persists in patients treated with ICSs and to evaluate the clinical features of patients with steroid-resistant airway type 2 inflammation.
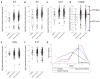
Methods: We used quantitative PCR to generate a composite metric of type 2 cytokine gene expression (type 2 gene mean [T2GM]) in induced sputum cells from healthy control subjects, patients with severe asthma receiving ICSs (n = 174), and patients with nonsevere asthma receiving ICSs (n = 85). We explored relationships between asthma outcomes and T2GM values and the utility of noninvasive biomarkers of airway T2GM.
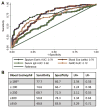
Results: Sputum cell T2GM values in asthmatic patients were significantly increased and remained high after treatment with intramuscular triamcinolone. We used the median T2GM value as a cutoff to classify steroid-treated type 2-low and steroid-resistant type 2-high (srT2-high) subgroups. Compared with patients with steroid-treated type 2-low asthma, those with srT2-high asthma were older and had more severe asthma. Blood eosinophil cell counts predicted srT2-high asthma when body mass index was less than 40 kg/m2 but not when it was 40 kg/m2 or greater, whereas blood IgE levels strongly predicted srT2-high asthma when age was less than 34 years but not when it was 34 years or greater.
Conclusion: Despite ICS therapy, many asthmatic patients have persistent airway type 2 inflammation (srT2-high asthma), and these patients are older and have more severe disease. Body weight and age modify the performance of blood-based biomarkers of airway type 2 inflammation.
Keywords: Severe asthma; biomarkers; steroid resistance; type 2 inflammation.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: M. C. Peters reports consultancy fees from Merck and Genentech. P. G. Woodruff reports consultancy fees from AstraZeneca, Theravance, Regeneron, Sanofi, Genentech, Novartis, and Janssen. B. D. Levy reports institutional National Institutes of Health (NIH) funding and consultancy fees from AstraZeneca, Merck, Pieris Pharmaceuticals, and Sanofi. E. Israel reports personal fees from AstraZeneca, Novartis, Philips Respironics, Regeneron Pharmaceuticals; fees from Research in Real Life (RiRL); personal fees and other from TEVA Specialty Pharmaceuticals; grants from Genentech; nonfinancial support from Boehringer Ingelheim, GlaxoSmithKline, Merck, Sunovion, and TEVA; grants from Sanofi; personal fees from Bird Rock Bio, Nuvelution Pharmaceuticals, and Vitaeris; grants from Boehringer Ingelheim; nonfinancial support from TEVA Specialty Pharmaceuticals; personal fees from Sanofi, Merck, Entrinsic Health Solutions, and Glaxo-SmithKline; other funds from Vorso; and personal fees from Pneuma Respiratory outside the submitted work. D. T. Mauger reports institutional grant funding from the NIH. S. C. Erzurum reports institutional NIH funding and has pending NIH grants and is Chair of the ABIM Pulmonary Disease Board and reports travel support from them. M. W. Johansson’s institution reports grant funding from the NIH, and he has grants pending from Hoffman-LaRoche, is a member of the Genentech Advisory Board, and received speaking fees from them. N. N. Jarjour reports consulting fees from Teva, Daiichi Sankyo, and AstraZeneca, and his institution reports NIH grant funding. A. M. Coverstone reports institutional NIH grants (U10 HL109257 and UL1 TR00448), and she has grant funding from Orbex (5U01HL3004502) and the Inner City Asthma Consortium (5UM1Al11427104 and 5UM1Al11427103). M. Castro reports grants from the NIH and ALA during the conduct of the study; personal fees from Aviragen, Boehringer-Ingelheim, Boston Scientific, Elsevier, Genentech, GlaxoSmithKline, Holaira, and Teva; and grants from Amgen, Boehringer-Ingelheim, Genentech, Gilead, GlaxoSmithKline, Invion, Medimmune, Sanofi-Aventis, and Vectura, all outside the submitted work. A. T. Hastie reports grants from the National Heart, Lung and Blood Institute (NHLBI) during the conduct of the study. E. R. Bleecker reports undertaking clinical trials through his employer, Wake Forest School of Medicine, and the University of Arizona and has also served as a paid consultant outside the submitted work. S. E. Wenzel has received consultancy fees from AstraZeneca (Anti-IL-5R/TSLP), Sanofi (anti–IL-4R), Genentech, and Teva (anti–IL-5) and her institution has grant funding pending from GlaxoSmithKline (anti–IL-33), Boehringer Ingelheim (anti–IL-23), and received funds from AstraZeneca (anti–IL-5R trials), GlaxoSmithK-line (anti–IL-5 trials), Sanofi (anti–IL-4R trials), and Novartis (CRTH2 antagonist trial). J. V. Fahy reports consultant fees from Boehringer Ingelheim, Dynavax, Medimmune, Theravance, Pieris, and Entrinsic Health Solutions; his institution has received NIH grant funding and has grants from NHLBI, Pfizer, Genentech, and Vitaeris, and the institution also received biomedical patents in which he was the named inventor (for the National Heart, Lung and Blood Institute Severe Asthma Research Program 3). The rest of the authors declare that they have no relevant conflicts of interest.
Figures


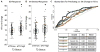
Comment in
-
Shedding light on corticosteroid-resistant type 2-high severe asthma.J Allergy Clin Immunol. 2019 Jan;143(1):89-90. doi: 10.1016/j.jaci.2018.11.002. Epub 2018 Nov 14. J Allergy Clin Immunol. 2019. PMID: 30445061 No abstract available.
References
-
- Gaga M, Zervas E, Chanez P. Update on severe asthma: what we know and what we need. Eur Respir Rev. 2009;18:58–65. - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HL109257/HL/NHLBI NIH HHS/United States
- K23 HL138303/HL/NHLBI NIH HHS/United States
- P01 HL107202/HL/NHLBI NIH HHS/United States
- U10 HL109250/HL/NHLBI NIH HHS/United States
- U10 HL109086/HL/NHLBI NIH HHS/United States
- U10 HL109168/HL/NHLBI NIH HHS/United States
- R01 HL080414/HL/NHLBI NIH HHS/United States
- U10 HL109172/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- K12 HL119997/HL/NHLBI NIH HHS/United States
- K24 HL137013/HL/NHLBI NIH HHS/United States
- R01 HL122531/HL/NHLBI NIH HHS/United States
- U19 AI077439/AI/NIAID NIH HHS/United States
- U10 HL109164/HL/NHLBI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U10 HL109152/HL/NHLBI NIH HHS/United States
- U10 HL109146/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

