Safety and Effectiveness of Direct Oral Anticoagulants Versus Vitamin K Antagonists: Pilot Implementation of a Near-Real-Time Monitoring Program in Italy
- PMID: 29525786
- PMCID: PMC5907561
- DOI: 10.1161/JAHA.117.008034
Safety and Effectiveness of Direct Oral Anticoagulants Versus Vitamin K Antagonists: Pilot Implementation of a Near-Real-Time Monitoring Program in Italy
Abstract
Background: Real-time monitoring is used to the ends of postmarketing observational research on newly marketed drugs. We implemented a pilot near-real-time monitoring program on the test case of oral anticoagulants. Specifically, we evaluated the safety and effectiveness of direct oral anticoagulants compared to vitamin K antagonists in nonvalvular atrial fibrillation secondary prevention during 2013-2015 in the Lazio Region, Italy.
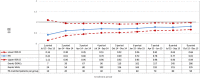
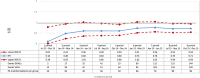
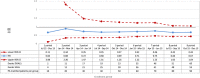
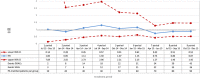
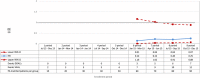
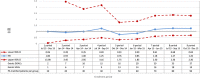
Methods and results: A cohort study was conducted using a sequential propensity-score-matched new user parallel-cohort design. Sequential analyses were performed using Cox models. Overall, 10 742 patients contributed to the analyses. Compared with vitamin K antagonists, direct oral anticoagulant use was associated with a reduction of all-cause mortality (0.81; 95% confidence interval [CI] 0.66-0.99), cardiovascular mortality (0.71; 95% CI 0.54-0.93), myocardial infarction (0.67; 95% CI 0.43-1.04), ischemic stroke (0.87; 95% CI 0.52-1.45), hemorrhagic stroke (0.25; 95% CI 0.07-0.88), and with a nonsignificant increase of gastrointestinal bleeding (1.26; 95% CI 0.69-2.30).
Conclusions: The present pilot study is a cornerstone to develop real-time monitoring for new drugs in our region.
Keywords: anticoagulant; comparative effectiveness; drug therapy; monitoring; pharmacoepidemiology; pilot; real‐world; surveillance.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

Similar articles
-
Safety and effectiveness of direct oral anticoagulants versus vitamin K antagonists: results from 3 Italian regions.Recenti Prog Med. 2019 Apr;110(4):195-202. doi: 10.1701/3154.31345. Recenti Prog Med. 2019. PMID: 31066365 English.
-
Comparative effectiveness and safety of direct oral anticoagulants versus vitamin K antagonists in nonvalvular atrial fibrillation: a Canadian multicentre observational cohort study.CMAJ Open. 2020 Dec 18;8(4):E877-E886. doi: 10.9778/cmajo.20200055. Print 2020 Oct-Dec. CMAJ Open. 2020. PMID: 33355273 Free PMC article.
-
New artificial intelligence prediction model using serial prothrombin time international normalized ratio measurements in atrial fibrillation patients on vitamin K antagonists: GARFIELD-AF.Eur Heart J Cardiovasc Pharmacother. 2020 Sep 1;6(5):301-309. doi: 10.1093/ehjcvp/pvz076. Eur Heart J Cardiovasc Pharmacother. 2020. PMID: 31821482 Free PMC article.
-
Periprocedural Outcomes of Direct Oral Anticoagulants Versus Warfarin in Nonvalvular Atrial Fibrillation.Circulation. 2018 Oct 2;138(14):1402-1411. doi: 10.1161/CIRCULATIONAHA.117.031457. Circulation. 2018. PMID: 29794081
-
Real-world Comparisons of Direct Oral Anticoagulants for Stroke Prevention in Asian Patients with Non-valvular Atrial Fibrillation: a Systematic Review and Meta-analysis.Cardiovasc Drugs Ther. 2019 Dec;33(6):701-710. doi: 10.1007/s10557-019-06910-z. Cardiovasc Drugs Ther. 2019. PMID: 31745687
Cited by
-
Effects of Carbamazepine and Phenytoin on Pharmacokinetics and Pharmacodynamics of Rivaroxaban.Pharmaceutics. 2020 Oct 30;12(11):1040. doi: 10.3390/pharmaceutics12111040. Pharmaceutics. 2020. PMID: 33143037 Free PMC article.
-
Comparing direct oral anticoagulants and vitamin K antagonist use in morbidly obese patients with venous thromboembolism: A single center retrospective cohort study.EJHaem. 2022 Mar 24;3(2):457-462. doi: 10.1002/jha2.418. eCollection 2022 May. EJHaem. 2022. PMID: 35846040 Free PMC article.
-
New Function for Safety Signal Monitoring in MID-NET®: The Case of an Anti-COVID-19 Drug.Clin Transl Sci. 2025 Apr;18(4):e70208. doi: 10.1111/cts.70208. Clin Transl Sci. 2025. PMID: 40171834 Free PMC article.
-
Cost-Effectiveness of Direct Non-Vitamin K Oral Anticoagulants Versus Vitamin K Antagonists for the Management of Patients with Non-Valvular Atrial Fibrillation Based on Available "Real-World" Evidence: The Italian National Health System Perspective.Clin Drug Investig. 2021 Mar;41(3):255-267. doi: 10.1007/s40261-021-01002-z. Epub 2021 Feb 15. Clin Drug Investig. 2021. PMID: 33587284 Free PMC article.
-
Sequential Epidemiological Analyses of Real-World Data: A Tool for Prospective Drug Safety Surveillance from the Rofecoxib Example.Drug Saf. 2025 May;48(5):489-502. doi: 10.1007/s40264-024-01512-7. Epub 2025 Jan 27. Drug Saf. 2025. PMID: 39869300 Free PMC article.
References
-
- FDA communication. Available at: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHum.... Accessed July 1, 2016.
-
- Eichler HG, Oye K, Baird LG, Abadie E, Brown J, Drum CL, Ferguson J, Garner S, Honig P, Hukkelhoven M, Lim JC, Lim R, Lumpkin MM, Neil G, O'Rourke B, Pezalla E, Shoda D, Seyfert‐Margolis V, Sigal EV, Sobotka J, Tan D, Unger TF, Hirsch G. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther. 2012;91:426–437. - PubMed
-
- Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network—improving the evidence of medical‐product safety. N Engl J Med. 2009;361:645–647. - PubMed
-
- Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the Sentinel System—a national resource for evidence development. N Engl J Med. 2011;364:498–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

