Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial
- PMID: 29525821
- PMCID: PMC5876881
- DOI: 10.1001/jama.2018.2444
Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial
Erratum in
-
Error in Figure.JAMA. 2018 Sep 4;320(9):938. doi: 10.1001/jama.2018.11635. JAMA. 2018. PMID: 30193257 Free PMC article. No abstract available.
Abstract
Importance: The effects of loading doses of statins on clinical outcomes in patients with acute coronary syndrome (ACS) and planned invasive management remain uncertain.
Objective: To determine if periprocedural loading doses of atorvastatin decrease 30-day major adverse cardiovascular events (MACE) in patients with ACS and planned invasive management.
Design, setting, and participants: Multicenter, double-blind, placebo-controlled, randomized clinical trial conducted at 53 sites in Brazil among 4191 patients with ACS evaluated with coronary angiography to proceed with a percutaneous coronary intervention (PCI) if anatomically feasible. Enrollment occurred between April 18, 2012, and October 6, 2017. Final follow-up for 30-day outcomes was on November 6, 2017.
Interventions: Patients were randomized to receive 2 loading doses of 80 mg of atorvastatin (n = 2087) or matching placebo (n = 2104) before and 24 hours after a planned PCI. All patients received 40 mg of atorvastatin for 30 days starting 24 hours after the second dose of study medication.
Main outcomes and measures: The primary outcome was MACE, defined as a composite of all-cause mortality, myocardial infarction, stroke, and unplanned coronary revascularization through 30 days.
Results: Among the 4191 patients (mean age, 61.8 [SD, 11.5] years; 1085 women [25.9%]) enrolled, 4163 (99.3%) completed 30-day follow-up. A total of 2710 (64.7%) underwent PCI, 333 (8%) underwent coronary artery bypass graft surgery, and 1144 (27.3%) had exclusively medical management. At 30 days, 130 patients in the atorvastatin group (6.2%) and 149 in the placebo group (7.1%) had a MACE (absolute difference, 0.85% [95% CI, -0.70% to 2.41%]; hazard ratio, 0.88; 95% CI, 0.69-1.11; P = .27). No cases of hepatic failure were reported; 3 cases of rhabdomyolysis were reported in the placebo group (0.1%) and 0 in the atorvastatin group.
Conclusions and relevance: Among patients with ACS and planned invasive management with PCI, periprocedural loading doses of atorvastatin did not reduce the rate of MACE at 30 days. These findings do not support the routine use of loading doses of atorvastatin among unselected patients with ACS and intended invasive management.
Trial registration: clinicaltrials.gov Identifier: NCT01448642.
Conflict of interest statement
Figures
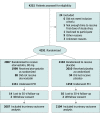

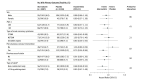
Comment in
-
Lipid Lowering in Acute Coronary Syndrome: Is Treatment Early Enough?JAMA. 2018 Apr 3;319(13):1325-1326. doi: 10.1001/jama.2018.2426. JAMA. 2018. PMID: 29525819 No abstract available.
-
SECURE PCI: how important can a subgroup analysis be?J Thorac Dis. 2018 Jun;10(Suppl 17):S2032-S2034. doi: 10.21037/jtd.2018.05.155. J Thorac Dis. 2018. PMID: 30023111 Free PMC article. No abstract available.
-
Early treatment with high-potency statins in patients with acute coronary syndrome-an example of personalized medicine.J Thorac Dis. 2018 Jun;10(Suppl 17):S2062-S2066. doi: 10.21037/jtd.2018.05.185. J Thorac Dis. 2018. PMID: 30023119 Free PMC article. No abstract available.
-
Timing of high intensity statin for acute coronary syndrome: how earlier initiation makes better?J Thorac Dis. 2018 Jul;10(Suppl 18):S2149-S2152. doi: 10.21037/jtd.2018.06.46. J Thorac Dis. 2018. PMID: 30123546 Free PMC article. No abstract available.
References
-
- Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. - PubMed
-
- Amsterdam EA, Wenger NK, Brindis RG, et al. . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228. - PubMed
-
- O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-e425. - PubMed
-
- Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA. 1998;279(20):1643-1650. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

