Monitoring and management of autoimmunity in multiple sclerosis patients treated with alemtuzumab: practical recommendations
- PMID: 29525836
- PMCID: PMC6182701
- DOI: 10.1007/s00415-018-8822-y
Monitoring and management of autoimmunity in multiple sclerosis patients treated with alemtuzumab: practical recommendations
Abstract
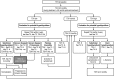
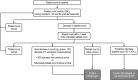
Alemtuzumab is a humanized anti-CD52 monoclonal antibody approved in more than 65 countries for the treatment of relapsing-remitting multiple sclerosis (RRMS). Compared with subcutaneous interferon-beta-1a, alemtuzumab significantly reduced clinical disease activity and the rate of brain volume loss, and improved disability outcomes in patients with active RRMS who were either treatment naive (CARE-MS I study) or who had an inadequate response (≥ 1 relapse after ≥ 6 months of treatment) to prior therapy (CARE-MS II study). Adverse events (AEs) associated with alemtuzumab include infusion-associated reactions, infections, and autoimmunity. The most commonly reported autoimmune AEs observed with alemtuzumab involve the thyroid gland, followed by immune thrombocytopenia and nephropathies. A monitoring program was designed and implemented to facilitate the early detection of autoimmune events to ensure timely and adequate management. The aim of this article is to provide physicians (including neurologists, general practitioners, endocrinologists, hematologists, and nephrologists who may be less familiar with the symptoms and treatment of autoimmune events), with practical real-world recommendations for the monitoring and management of autoimmunity associated with alemtuzumab treatment.
Keywords: Alemtuzumab; Autoimmunity; Immune thrombocytopenia; Multiple sclerosis; Nephropathy; Thyroid disorder.
Conflict of interest statement
Human and animal rights
All human studies reported herein were approved by the appropriate ethics committee and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its amendments.
Informed consent
All persons provided their informed consent prior to their inclusion in the studies.
Conflicts of interest
Virginia Devonshire has acted as a Principal Clinical Trial Investigator (Biogen, EMD Serono, MedDay, Novartis), participated in advisory boards (Biogen, EMD Serono, Novartis, Roche, Sanofi, and Teva), and received research financial support (Biogen, EMD Serono, Novartis). Gerald da Roza, Richard Phillips and Hilary Wass report no conflicts of interest. Peter Senior has received consulting and/or speaking fees and grant/research support (Sanofi).
Figures


References
-
- De Mercanti S, Rolla S, Cucci A, Bardina V, Cocco E, Vladic A, Soldo-Butkovic S, Habek M, Adamec I, Horakova D, Annovazzi P, Novelli F, Durelli L, Clerico M. Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm. 2016;3:e194. doi: 10.1212/NXI.0000000000000194. - DOI - PMC - PubMed
-
- Zhang X, Tao Y, Chopra M, Ahn M, Marcus KL, Choudhary N, Zhu H, Markovic-Plese S. Differential reconstitution of T cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 2013;191:5867–5874. doi: 10.4049/jimmunol.1301926. - DOI - PubMed
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

