Excitonic Interactions in Bacteriochlorin Homo-Dyads Enable Charge Transfer: A New Approach to the Artificial Photosynthetic Special Pair
- PMID: 29526105
- PMCID: PMC6422163
- DOI: 10.1021/acs.jpcb.8b02123
Excitonic Interactions in Bacteriochlorin Homo-Dyads Enable Charge Transfer: A New Approach to the Artificial Photosynthetic Special Pair
Abstract
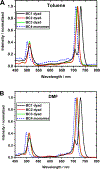
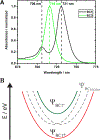
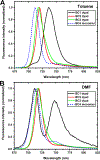
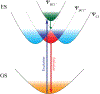
Excitonically coupled bacteriochlorin (BC) dimers constitute a primary electron donor (special pair) in bacterial photosynthesis and absorbing units in light-harvesting antenna. However, the exact nature of the excited state of these dyads is still not fully understood. Here, we report a detailed spectroscopic and computational investigation of a series of symmetrical bacteriochlorin dimers, where the bacteriochlorins are connected either directly or by a phenylene bridge of variable length. The excited state of these dyads is quenched in high-dielectric solvents, which we attribute to photoinduced charge transfer. The mixing of charge transfer with the excitonic state causes accelerated (within 41 ps) decay of the excited state for the directly linked dyad, which is reduced by orders of magnitude with each additional phenyl ring separating the bacteriochlorins. These results highlight the origins of the excited-state dynamics in symmetric BC dyads and provide a new model for studying the primary processes in photosynthesis and for the development of artificial, biomimetic systems for solar energy conversion.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures




Similar articles
-
Photoinduced charge transfer in Zn(II) and Au(III)-ligated symmetric and asymmetric bacteriochlorin dyads: A computational study.J Chem Phys. 2020 Oct 7;153(13):134111. doi: 10.1063/5.0023609. J Chem Phys. 2020. PMID: 33032416
-
Synthesis and excited-state photodynamics of a chlorin-bacteriochlorin dyad--through-space versus through-bond energy transfer in tetrapyrrole arrays.Photochem Photobiol. 2008 May-Jun;84(3):786-801. doi: 10.1111/j.1751-1097.2007.00258.x. Epub 2008 Jan 15. Photochem Photobiol. 2008. PMID: 18208458
-
Chlorin-bacteriochlorin energy-transfer dyads as prototypes for near-infrared molecular imaging probes: controlling charge-transfer and fluorescence properties in polar media.Photochem Photobiol. 2009 Jul-Aug;85(4):909-20. doi: 10.1111/j.1751-1097.2008.00532.x. Epub 2009 Feb 13. Photochem Photobiol. 2009. PMID: 19222800
-
Porphyrin-fullerene linked systems as artificial photosynthetic mimics.Org Biomol Chem. 2004 May 21;2(10):1425-33. doi: 10.1039/b403024a. Epub 2004 Apr 26. Org Biomol Chem. 2004. PMID: 15136797 Review.
-
Long-lived charge separation and applications in artificial photosynthesis.Acc Chem Res. 2014 May 20;47(5):1455-64. doi: 10.1021/ar400200u. Epub 2014 May 5. Acc Chem Res. 2014. PMID: 24793793 Review.
Cited by
-
De novo design of proteins housing excitonically coupled chlorophyll special pairs.Nat Chem Biol. 2024 Jul;20(7):906-915. doi: 10.1038/s41589-024-01626-0. Epub 2024 Jun 3. Nat Chem Biol. 2024. PMID: 38831036 Free PMC article.
-
De novo design of energy transfer proteins housing excitonically coupled chlorophyll special pairs.Res Sq [Preprint]. 2023 Apr 21:rs.3.rs-2736786. doi: 10.21203/rs.3.rs-2736786/v1. Res Sq. 2023. Update in: Nat Chem Biol. 2024 Jul;20(7):906-915. doi: 10.1038/s41589-024-01626-0. PMID: 37131790 Free PMC article. Updated. Preprint.
-
Charge transfer as a mechanism for chlorophyll fluorescence concentration quenching.Proc Natl Acad Sci U S A. 2023 Jan 31;120(5):e2210811120. doi: 10.1073/pnas.2210811120. Epub 2023 Jan 23. Proc Natl Acad Sci U S A. 2023. PMID: 36689657 Free PMC article.
References
-
- Advances in Photosynthesis and Respiration. In Chlorophylls and Bacteriochlorophylls: Biochemistry, BiophysicsFunctions and Applications; Grimm B, Ed.; Springer: Dordrecht, 2006; Vol. 25.
-
- Blankenship RE Molecular Mechanisms of Photosynthesis; John Wiley & Sons, 2014.
-
- Zinth W; Wachtveitl J The First Picoseconds in Bacterial Photosynthesis—Ultrafast Electron Transfer for the Efficient Conversion of Light Energy. ChemPhysChem 2005, 6, 871–880. - PubMed
-
- Mirkovic T; Ostroumov EE; Anna JM; van Grondelle R; Govindjee; Scholes GD Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. - PubMed
-
- Croce R; van Amerongen H Natural Strategies for Photosynthetic Light Harvesting. Nat. Chem. Biol. 2014, 10, 492–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

