Lack of Type 2 Innate Lymphoid Cells Promotes a Type I-Driven Enhanced Immune Response in Contact Hypersensitivity
- PMID: 29526762
- PMCID: PMC6117454
- DOI: 10.1016/j.jid.2018.03.001
Lack of Type 2 Innate Lymphoid Cells Promotes a Type I-Driven Enhanced Immune Response in Contact Hypersensitivity
Abstract
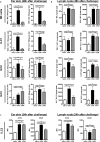
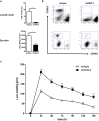
Allergic contact dermatitis and its animal model, contact hypersensitivity, are T-cell-mediated inflammatory skin diseases that require activation of the innate immune system. Here we investigate the role of innate lymphoid cells (ILCs) during the elicitation phase of 2,4,6-trinitrochlorobenzene-induced contact hypersensitivity using EomesGfp/+ x Rorc(γt)-CreTg x Rosa26RYfp/+ reporter mice. Ear swelling responses, cutaneous ILC numbers, and cytokine production were determined at different time points. Functional analyses were performed in a CD90.1/.2 congenic adoptive transfer model that allowed selective antibody-mediated depletion of ILCs before hapten challenge, and in Rorasg/floxIl7rCre/+ mice, which lack ILC2. Hapten challenge induced early increases of natural killer cells in skin and ear draining lymph nodes corresponding to the peak ear swelling response. In contrast, ILC1, 2, and 3 showed a delayed increase in numbers corresponding to the contact hypersensitivity resolution phase. Hapten challenge induced increased marker cytokines in all ILC subtypes and an activated phenotype in ILC2. Depletion of all ILC resulted in a significantly enhanced ear swelling response. Similarly, ILC2-deficient mice (Rorasg/floxIl7rCre/+) displayed increased ear swelling responses on hapten challenge, suggesting that ILC2 act as negative regulators in the type 1-dominated immune response of contact hypersensitivity.
Copyright © 2018 MRC Lab of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

