Adjuvant immunotherapy for cancer: both dendritic cell-priming and check-point inhibitor blockade are required for immunotherapy
- PMID: 29526974
- PMCID: PMC5909060
- DOI: 10.2183/pjab.94.011
Adjuvant immunotherapy for cancer: both dendritic cell-priming and check-point inhibitor blockade are required for immunotherapy
Abstract
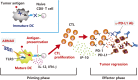
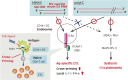
The immune system eliminates advanced cancer when treated with programmed cell death protein-1 (PD-1) or its ligand (PD-L1) blockade, but PD-1 therapy is effective in only ∼20% of patients with solid cancer. The PD-1 antibody mainly acts on the effector phase of cytotoxic T lymphocytes (CTLs) in tumors but induces no activation of the priming phase of antigen-presenting dendritic cells (DCs). It is reasonable that both DC-priming and PD-1/L1 blocking are mandatory for efficient CTL-mediated tumor cytolysis. For DC-priming, a therapeutic vaccine containing Toll-like receptor (TLR) agonists, namely a priming adjuvant, is a good candidate; however, a means for DC-targeting by TLR adjuvant therapy remains to be developed. TLR adjuvants usually harbor cytokine toxicity, which is a substantial barrier against drug approval. Here, we discuss the functional properties of current TLR adjuvants for cancer immunotherapy and introduce a TLR3-specific adjuvant (ARNAX) that barely induces cytokinemia in mouse models.
Keywords: PD-1/L1 blockade; TLR3; adjuvant; cancer immunotherapy; polyI:C.
Figures
Similar articles
-
A TLR3-Specific Adjuvant Relieves Innate Resistance to PD-L1 Blockade without Cytokine Toxicity in Tumor Vaccine Immunotherapy.Cell Rep. 2017 May 30;19(9):1874-1887. doi: 10.1016/j.celrep.2017.05.015. Cell Rep. 2017. PMID: 28564605
-
A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy.Adv Drug Deliv Rev. 2019 Jul;147:37-43. doi: 10.1016/j.addr.2019.07.008. Epub 2019 Jul 11. Adv Drug Deliv Rev. 2019. PMID: 31302192 Review.
-
Vaccine immunotherapy with ARNAX induces tumor-specific memory T cells and durable anti-tumor immunity in mouse models.Cancer Sci. 2018 Jul;109(7):2119-2129. doi: 10.1111/cas.13649. Epub 2018 Jun 16. Cancer Sci. 2018. PMID: 29791768 Free PMC article.
-
Targeting Toll-like receptor 3 in dendritic cells for cancer immunotherapy.Expert Opin Biol Ther. 2020 Aug;20(8):937-946. doi: 10.1080/14712598.2020.1749260. Epub 2020 Apr 7. Expert Opin Biol Ther. 2020. PMID: 32223572 Review.
-
Type I Interferon-Independent Dendritic Cell Priming and Antitumor T Cell Activation Induced by a Mycoplasma fermentans Lipopeptide.Front Immunol. 2018 Mar 14;9:496. doi: 10.3389/fimmu.2018.00496. eCollection 2018. Front Immunol. 2018. PMID: 29593736 Free PMC article.
Cited by
-
Two Modes of Th1 Polarization Induced by Dendritic-Cell-Priming Adjuvant in Vaccination.Cells. 2023 May 29;12(11):1504. doi: 10.3390/cells12111504. Cells. 2023. PMID: 37296625 Free PMC article. Review.
-
Adjuvant Effect of Toll-Like Receptor 9 Activation on Cancer Immunotherapy Using Checkpoint Blockade.Front Immunol. 2020 May 29;11:1075. doi: 10.3389/fimmu.2020.01075. eCollection 2020. Front Immunol. 2020. PMID: 32547560 Free PMC article. Review.
-
Cytokine-induced killer cells co-cultured with non-cell derived targeting peptide-loaded dendritic cells induce a specific antitumor response.Cancer Biol Ther. 2019;20(5):720-728. doi: 10.1080/15384047.2018.1564561. Epub 2019 Feb 19. Cancer Biol Ther. 2019. PMID: 30777479 Free PMC article.
-
Dendritic cells transduced with glioma-expressed antigen 2 recombinant adenovirus induces specific cytotoxic lymphocyte response and anti-tumor effect in mice.J Inflamm (Lond). 2020 Jan 31;17:3. doi: 10.1186/s12950-020-0239-6. eCollection 2020. J Inflamm (Lond). 2020. PMID: 32021567 Free PMC article.
-
Monophosphoryl lipid A-induced activation of plasmacytoid dendritic cells enhances the anti-cancer effects of anti-PD-L1 antibodies.Cancer Immunol Immunother. 2021 Mar;70(3):689-700. doi: 10.1007/s00262-020-02715-4. Epub 2020 Sep 9. Cancer Immunol Immunother. 2021. PMID: 32902663 Free PMC article.
References
-
- Burnet F.M. (1970) The concept of immunological surveillance. Prog. Exp. Tumor Res. 13, 1–27. - PubMed
-
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., et al. (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647. - PubMed
-
- Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998. doi:10.1038/ni1102-991. - PubMed
-
- Hanahan D., Weinberg R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. doi:10.1016/j.cell.2011.02.013. - PubMed
-
- Desmet C.J., Ishii K.J. (2012) Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 12, 479–491. doi:10.1038/nri3247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

