Toward Strong and Tough Glass and Ceramic Scaffolds for Bone Repair
- PMID: 29527148
- PMCID: PMC5844579
- DOI: 10.1002/adfm.201301121
Toward Strong and Tough Glass and Ceramic Scaffolds for Bone Repair
Abstract
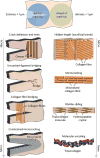
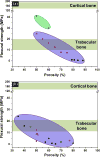
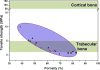
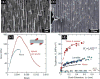
The need for implants to repair large bone defects is driving the development of porous synthetic scaffolds with the requisite mechanical strength and toughness in vivo. Recent developments in the use of design principles and novel fabrication technologies are paving the way to create synthetic scaffolds with promising potential for reconstituting bone in load-bearing sites. This article reviews the state of the art in the design and fabrication of bioactive glass and ceramic scaffolds that have improved mechanical properties for structural bone repair. Scaffolds with anisotropic and periodic structures can be prepared with compressive strengths comparable to human cortical bone (100-150 MPa), while scaffolds with an isotropic structure typically have strengths in the range of trabecular bone (2-12 MPa). However, the mechanical response of bioactive glass and ceramic scaffolds in multiple loading modes such as flexure and torsion - as well as their mechanical reliability, fracture toughness, and fatigue resistance - has received little attention. Inspired by the designs of natural materials such as cortical bone and nacre, glass-ceramic and inorganic/polymer composite scaffolds created with extrinsic toughening mechanisms are showing potential for both high strength and mechanical reliability. Future research should include improved designs that provide strong scaffolds with microstructures conducive to bone ingrowth, and evaluation of these scaffolds in large animal models for eventual translation into clinical applications.
Keywords: Bioactive glass and ceramics; bone tissue engineering; mechanical strength; porous scaffolds; reliability.
Figures







Similar articles
-
Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives.Mater Sci Eng C Mater Biol Appl. 2011 Oct 10;31(7):1245-1256. doi: 10.1016/j.msec.2011.04.022. Mater Sci Eng C Mater Biol Appl. 2011. PMID: 21912447 Free PMC article.
-
Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects.Sci Rep. 2016 Jan 19;6:19468. doi: 10.1038/srep19468. Sci Rep. 2016. PMID: 26782020 Free PMC article.
-
Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration.Acta Biomater. 2011 Oct;7(10):3547-54. doi: 10.1016/j.actbio.2011.06.030. Epub 2011 Jun 28. Acta Biomater. 2011. PMID: 21745606 Free PMC article.
-
Toughening and functionalization of bioactive ceramic and glass bone scaffolds by biopolymer coatings and infiltration: a review of the last 5 years.Expert Rev Med Devices. 2015 Jan;12(1):93-111. doi: 10.1586/17434440.2015.958075. Epub 2014 Oct 21. Expert Rev Med Devices. 2015. PMID: 25331196 Review.
-
Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109895. doi: 10.1016/j.msec.2019.109895. Epub 2019 Jun 16. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31500047 Review.
Cited by
-
Exquisite design of injectable Hydrogels in Cartilage Repair.Theranostics. 2020 Aug 2;10(21):9843-9864. doi: 10.7150/thno.46450. eCollection 2020. Theranostics. 2020. PMID: 32863963 Free PMC article. Review.
-
Overview of porous magnesium-based scaffolds: development, properties and biomedical applications.Mater Futur. 2025 Mar 1;4(1):012401. doi: 10.1088/2752-5724/ad9493. Epub 2025 Jan 2. Mater Futur. 2025. PMID: 39758543 Free PMC article. Review.
-
Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect.Acta Biomater. 2015 Jul;20:113-119. doi: 10.1016/j.actbio.2015.04.007. Epub 2015 Apr 11. Acta Biomater. 2015. PMID: 25871536 Free PMC article.
-
Incorporating the Antioxidant Fullerenol into Calcium Phosphate Bone Cements Increases Cellular Osteogenesis without Compromising Physical Cement Characteristics.Adv Eng Mater. 2023 Sep;25(17):2300301. doi: 10.1002/adem.202300301. Epub 2023 Jun 24. Adv Eng Mater. 2023. PMID: 37982016 Free PMC article.
-
Prefabricated 3D-Printed Tissue-Engineered Bone for Mandibular Reconstruction: A Preclinical Translational Study in Primate.ACS Biomater Sci Eng. 2021 Dec 13;7(12):5727-5738. doi: 10.1021/acsbiomaterials.1c00509. Epub 2021 Nov 22. ACS Biomater Sci Eng. 2021. PMID: 34808042 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
