Successful Low-Cost Scaffold-Free Cartilage Tissue Engineering Using Human Cartilage Progenitor Cell Spheroids Formed by Micromolded Nonadhesive Hydrogel
- PMID: 29527227
- PMCID: PMC5750468
- DOI: 10.1155/2017/7053465
Successful Low-Cost Scaffold-Free Cartilage Tissue Engineering Using Human Cartilage Progenitor Cell Spheroids Formed by Micromolded Nonadhesive Hydrogel
Abstract
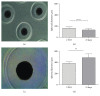
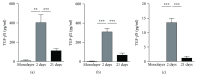
The scaffold-free tissue engineering using spheroids is pointed out as an approach for optimizing the delivery system of cartilage construct. In this study, we aimed to evaluate the micromolded nonadhesive hydrogel (MicroTissues®) for spheroid compaction (2-day culture) and spontaneous chondrogenesis (21-day culture) using cartilage progenitors cells (CPCs) from human nasal septum without chondrogenic stimulus. CPC spheroids showed diameter stability (486 μm ± 65), high percentage of viable cells (88.1 ± 2.1), and low percentage of apoptotic cells (2.3%). After spheroid compaction, the synthesis of TGF-β1, TGF-β2, and TGF-β3 was significantly higher compared to monolayer (p < 0.005). Biomechanical assay revealed that the maximum forces applied to spheroids after chondrogenesis were 2.6 times higher than for those cultured for 2 days. After spontaneous chondrogenesis, CPC spheroids were entirely positive for N-cadherin, collagen type II and type VI, and aggrecan and chondroitin sulfate. Comparing to monolayer, the expression of SOX5 and SOX6 genes analyzed by qPCR was significantly upregulated (p < 0.01). Finally, we observed the capacity of CPC spheroids starting to fuse. To the best of our knowledge, this is the first time in the scientific literature that human CPC spheroids were formed by micromolded nonadhesive hydrogel, achieving a successful scaffold-free cartilage engineering without chondrogenic stimulus (low cost).
Figures




Similar articles
-
Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks.World J Stem Cells. 2020 Feb 26;12(2):110-122. doi: 10.4252/wjsc.v12.i2.110. World J Stem Cells. 2020. PMID: 32184936 Free PMC article. Review.
-
A Scaffold- and Serum-Free Method to Mimic Human Stable Cartilage Validated by Secretome.Tissue Eng Part A. 2021 Mar;27(5-6):311-327. doi: 10.1089/ten.TEA.2018.0311. Epub 2019 May 2. Tissue Eng Part A. 2021. PMID: 30734654
-
Small spheroids for head and neck cartilage tissue engineering.Sci Rep. 2024 Dec 30;14(1):32114. doi: 10.1038/s41598-024-83847-w. Sci Rep. 2024. PMID: 39738737 Free PMC article.
-
Amniotic fluid MSCs for scaffold-free cartilage repair: spheroid fusion and chondrogenic microtissue development.Future Sci OA. 2025 Dec;11(1):2476922. doi: 10.1080/20565623.2025.2476922. Epub 2025 Jun 11. Future Sci OA. 2025. PMID: 40497630 Free PMC article.
-
Chondrogenic differentiation of mesenchymal stem cells and its clinical applications.Yonsei Med J. 2004 Jun 30;45 Suppl:41-7. doi: 10.3349/ymj.2004.45.Suppl.41. Yonsei Med J. 2004. PMID: 15250049 Review.
Cited by
-
Extracellular Matrix Determines Biomechanical Properties of Chondrospheres during Their Maturation In Vitro.Cartilage. 2020 Oct;11(4):521-531. doi: 10.1177/1947603518798890. Epub 2018 Sep 15. Cartilage. 2020. PMID: 30221989 Free PMC article.
-
Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks.World J Stem Cells. 2020 Feb 26;12(2):110-122. doi: 10.4252/wjsc.v12.i2.110. World J Stem Cells. 2020. PMID: 32184936 Free PMC article. Review.
-
Enhanced articular cartilage regeneration using costal chondrocyte-derived scaffold-free tissue engineered constructs with ascorbic acid treatment.J Orthop Translat. 2024 Mar 22;45:140-154. doi: 10.1016/j.jot.2024.02.005. eCollection 2024 Mar. J Orthop Translat. 2024. PMID: 38559899 Free PMC article.
-
Mimicking lipolytic, adipogenic, and secretory capacities of human subcutaneous adipose tissue by spheroids from distinct subpopulations of adipose stromal/stem cells.Front Cell Dev Biol. 2023 Sep 29;11:1219218. doi: 10.3389/fcell.2023.1219218. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37842092 Free PMC article.
-
Tissue engineering applications in otolaryngology-The state of translation.Laryngoscope Investig Otolaryngol. 2020 Jun 19;5(4):630-648. doi: 10.1002/lio2.416. eCollection 2020 Aug. Laryngoscope Investig Otolaryngol. 2020. PMID: 32864434 Free PMC article. Review.
References
-
- Fujie H., Nansai R., Ando W., et al. Zone-specific integrated cartilage repair using a scaffold-free tissue engineered construct derived from allogenic synovial mesenchymal stem cells: biomechanical and histological assessments. Journal of Biomechanics. 2015;48(15):4101–4108. doi: 10.1016/j.jbiomech.2015.10.015. - DOI - PubMed
-
- Ishihara K., Nakayama K., Akieda S., Matsuda S., Iwamoto Y. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. Journal of Orthopaedic Surgery Research. 2014;9(98):1–10. doi: 10.1186/s13018-014-0098-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

