Alkaline sphingomyelinase (NPP7) in hepatobiliary diseases: A field that needs to be closely studied
- PMID: 29527260
- PMCID: PMC5838443
- DOI: 10.4254/wjh.v10.i2.246
Alkaline sphingomyelinase (NPP7) in hepatobiliary diseases: A field that needs to be closely studied
Abstract
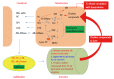
Alkaline sphingomyelinase cleaves phosphocholine from sphingomyelin, platelet-activating factor, lysophosphatidylcholine, and less effectively phosphatidylcholine. The enzyme shares no structure similarities with acid or neutral sphingomyelinase but belongs to ecto-nucleotide pyrophosphatase/phosphodiesterase (NPP) family and therefore is also called NPP7 nowadays. The enzyme is expressed in the intestinal mucosa in many species and additionally in human liver. The enzyme in the intestinal tract has been extensively studied but not that in human liver. Studies on intestinal alkaline sphingomyelinase show that it inhibits colonic tumorigenesis and inflammation, hydrolyses dietary sphingomyelin, and stimulates cholesterol absorption. The review aims to summarize the current knowledge on liver alkaline sphingomyelinase in human and strengthen the necessity for close study on this unique human enzyme in hepatobiliary diseases.
Keywords: Alkaline sphingomyelinase; Autotaxin; Cholangiocarcinoma; Gallstone; Liver diseases; Nucleotide pyrophosphatase/phosphodiesterase 7; Platelet-activating factor; Sphingomyelin.
Conflict of interest statement
Conflict-of-interest statement: No conflict-of-interest to be disclosed.
Figures
Similar articles
-
Changes of activity and isoforms of alkaline sphingomyelinase (nucleotide pyrophosphatase phosphodiesterase 7) in bile from patients undergoing endoscopic retrograde cholangiopancreatography.BMC Gastroenterol. 2014 Aug 7;14:138. doi: 10.1186/1471-230X-14-138. BMC Gastroenterol. 2014. PMID: 25100243 Free PMC article.
-
Computational identification and experimental characterization of substrate binding determinants of nucleotide pyrophosphatase/phosphodiesterase 7.BMC Biochem. 2011 Dec 16;12:65. doi: 10.1186/1471-2091-12-65. BMC Biochem. 2011. PMID: 22177013 Free PMC article.
-
Alkaline sphingomyelinase: an old enzyme with novel implications.Biochim Biophys Acta. 2006 Mar;1761(3):281-91. doi: 10.1016/j.bbalip.2006.03.007. Epub 2006 Mar 30. Biochim Biophys Acta. 2006. PMID: 16631405 Review.
-
Understanding the molecular activity of alkaline sphingomyelinase (NPP7) by computer modeling.Biochemistry. 2010 Oct 26;49(42):9096-105. doi: 10.1021/bi101069u. Biochemistry. 2010. PMID: 20839774
-
Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5'-nucleotidase, and alkaline phosphatase inhibitors.Med Res Rev. 2014 Jul;34(4):703-43. doi: 10.1002/med.21302. Epub 2013 Sep 23. Med Res Rev. 2014. PMID: 24115166 Review.
Cited by
-
A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis.Int J Mol Sci. 2021 May 28;22(11):5793. doi: 10.3390/ijms22115793. Int J Mol Sci. 2021. PMID: 34071409 Free PMC article. Review.
-
Autotaxin activity predicts transplant-free survival in primary sclerosing cholangitis.Sci Rep. 2019 Jun 11;9(1):8450. doi: 10.1038/s41598-019-44762-7. Sci Rep. 2019. PMID: 31186435 Free PMC article.
-
Sphingomyelinases and Liver Diseases.Biomolecules. 2020 Oct 30;10(11):1497. doi: 10.3390/biom10111497. Biomolecules. 2020. PMID: 33143193 Free PMC article. Review.
-
Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology.Front Physiol. 2024 Jan 3;14:1229108. doi: 10.3389/fphys.2023.1229108. eCollection 2023. Front Physiol. 2024. PMID: 38235387 Free PMC article. Review.
-
Metabolomic profiles of incident gallstone disease.BMJ Open Gastroenterol. 2024 Aug 28;11(1):e001417. doi: 10.1136/bmjgast-2024-001417. BMJ Open Gastroenterol. 2024. PMID: 39209332 Free PMC article.
References
-
- Nilsson A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969;176:339–347. - PubMed
-
- Duan RD, Nyberg L, Nilsson A. Alkaline sphingomyelinase activity in rat gastrointestinal tract: distribution and characteristics. Biochim Biophys Acta. 1995;1259:49–55. - PubMed
-
- Wu J, Cheng Y, Palmberg C, Bergman T, Nilsson A, Duan RD. Cloning of alkaline sphingomyelinase from rat intestinal mucosa and adjusting of the hypothetical protein XP_221184 in GenBank. Biochim Biophys Acta. 2005;1687:94–102. - PubMed
-
- Cheng Y, Nilsson A, Tömquist E, Duan RD. Purification, characterization, and expression of rat intestinal alkaline sphingomyelinase. J Lipid Res. 2002;43:316–324. - PubMed
-
- Duan RD, Bergman T, Xu N, Wu J, Cheng Y, Duan J, Nelander S, Palmberg C, Nilsson A. Identification of human intestinal alkaline sphingomyelinase as a novel ecto-enzyme related to the nucleotide phosphodiesterase family. J Biol Chem. 2003;278:38528–38536. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

