Homologous recombination mediates stable Fah gene integration and phenotypic correction in tyrosinaemia mouse-model
- PMID: 29527263
- PMCID: PMC5838446
- DOI: 10.4254/wjh.v10.i2.277
Homologous recombination mediates stable Fah gene integration and phenotypic correction in tyrosinaemia mouse-model
Abstract
Aim: To stably correct tyrosinaemia in proliferating livers of fumarylacetoacetate-hydrolase knockout (Fah-/-) mice by homologous-recombination-mediated targeted addition of the Fah gene.
Methods: C57BL/6 Fah∆exon5 mice served as an animal model for human tyrosinaemia type 1 in our study. The vector was created by amplifying human Fah cDNA including the TTR promoter from a lentivirus plasmid as described. The Fah expression cassette was flanked by homologous arms (620 bp and 749 bp long) of the Rosa26 gene locus. Mice were injected with 2.1 × 108 VP of this vector (rAAV8-ROSA26.HAL-TTR.Fah-ROSA26.HAR) via the tail vein. Mice in the control group were injected with 2.1 × 108 VP of a similar vector but missing the homologous arms (rAAV8-TTR.Fah). Primary hepatocytes from Fah-/- recipient mice, treated with our vectors, were isolated and 1 × 106 hepatocytes were transplanted into secondary Fah-/- recipient mice by injection into the spleen. Upon either vector application or hepatocyte transplantation NTBC treatment was stopped in recipient mice.
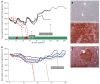
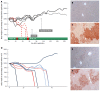
Results: Here, we report successful HR-mediated genome editing by integration of a Fah gene expression cassette into the "safe harbour locus" Rosa26 by recombinant AAV8. Both groups of mice showed long-term survival, weight gain and FAH positive clusters as determined by immunohistochemistry analysis of liver sections in the absence of NTBC treatment. In the group of C57BL/6 Fah∆exon5 mice, which have been transplanted with hepatocytes from a mouse injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26.HAR 156 d before, 6 out of 6 mice showed long-term survival, weight gain and FAH positive clusters without need for NTBC treatment. In contrast only 1 out 5 mice, who received hepatocytes from rAAV8-TTR.Fah treated mice, survived and showed few and smaller FAH positive clusters. These results demonstrate that homologous recombination-mediated Fah gene transfer corrects the phenotype in a mouse model of human tyrosinaemia type 1 (Fah-/- mice) and is long lasting in a proliferating state of the liver as shown by withdrawal of NTBC treatment and serial transplantation of isolated hepatocytes from primary Fah-/- recipient mice into secondary Fah-/- recipient mice. This long term therapeutic efficacy is clearly superior to our control mice treated with episomal rAAV8 gene therapy approach.
Conclusion: HR-mediated rAAV8 gene therapy provides targeted transgene integration and phenotypic correction in Fah-/- mice with superior long-term efficacy compared to episomal rAAV8 therapy in proliferating livers.
Keywords: AAV8; Gene therapy; Liver based metabolic disease; Paediatric liver disease; ROSA26; Targeted integration.
Conflict of interest statement
Conflict-of-interest statement: There are no conflicts of interest for any of the authors regarding this work.
Figures



Similar articles
-
Curative Ex Vivo Hepatocyte-Directed Gene Editing in a Mouse Model of Hereditary Tyrosinemia Type 1.Hum Gene Ther. 2018 Nov;29(11):1315-1326. doi: 10.1089/hum.2017.252. Epub 2018 Jun 22. Hum Gene Ther. 2018. PMID: 29764210 Free PMC article.
-
Sleeping Beauty transposon system is a reliable gene delivery tool for hereditary tyrosinaemia type 1 disease gene therapy: size of the foreign gene decides the timing of stable integration into the host chromosomes.J Int Med Res. 2012;40(5):1850-9. doi: 10.1177/030006051204000523. J Int Med Res. 2012. PMID: 23206466
-
Generation of healthy mice from gene-corrected disease-specific induced pluripotent stem cells.PLoS Biol. 2011 Jul;9(7):e1001099. doi: 10.1371/journal.pbio.1001099. Epub 2011 Jul 12. PLoS Biol. 2011. PMID: 21765802 Free PMC article.
-
Tyrosinaemia type I and apoptosis of hepatocytes and renal tubular cells.J Inherit Metab Dis. 2002 May;25(3):227-34. doi: 10.1023/a:1015646400182. J Inherit Metab Dis. 2002. PMID: 12137232 Review.
-
Fah Knockout Animals as Models for Therapeutic Liver Repopulation.Adv Exp Med Biol. 2017;959:215-230. doi: 10.1007/978-3-319-55780-9_20. Adv Exp Med Biol. 2017. PMID: 28755199 Review.
Cited by
-
mRNA-based therapy proves superior to the standard of care for treating hereditary tyrosinemia 1 in a mouse model.Mol Ther Methods Clin Dev. 2022 Jul 15;26:294-308. doi: 10.1016/j.omtm.2022.07.006. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 35949297 Free PMC article.
-
CRISPR-Cas9-mediated somatic correction of a one-base deletion in the Ugt1a gene ameliorates hyperbilirubinemia in Crigler-Najjar syndrome mice.Mol Ther Methods Clin Dev. 2023 Nov 19;31:101161. doi: 10.1016/j.omtm.2023.101161. eCollection 2023 Dec 14. Mol Ther Methods Clin Dev. 2023. PMID: 38094199 Free PMC article.
-
Ex Vivo/In vivo Gene Editing in Hepatocytes Using "All-in-One" CRISPR-Adeno-Associated Virus Vectors with a Self-Linearizing Repair Template.iScience. 2020 Jan 24;23(1):100764. doi: 10.1016/j.isci.2019.100764. Epub 2019 Dec 12. iScience. 2020. PMID: 31887661 Free PMC article.
-
Modern Approaches to Mouse Genome Editing Using the CRISPR-Cas Toolbox and Their Applications in Functional Genomics and Translational Research.Adv Exp Med Biol. 2023;1429:13-40. doi: 10.1007/978-3-031-33325-5_2. Adv Exp Med Biol. 2023. PMID: 37486514 Review.
-
Genome editing technologies to treat rare liver diseases.Transl Gastroenterol Hepatol. 2020 Apr 5;5:23. doi: 10.21037/tgh.2019.10.10. eCollection 2020. Transl Gastroenterol Hepatol. 2020. PMID: 32258527 Free PMC article. Review.
References
-
- Junge N, Mingozzi F, Ott M, Baumann U. Adeno-associated virus vector-based gene therapy for monogenetic metabolic diseases of the liver. J Pediatr Gastroenterol Nutr. 2015;60:433–440. - PubMed
-
- Pañeda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ, Beattie SG, Twisk J, van Deventer S, Prieto J, et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20:908–917. - PubMed
-
- Hasbrouck NC, High KA. AAV-mediated gene transfer for the treatment of hemophilia B: problems and prospects. Gene Ther. 2008;15:870–875. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

