Study for every other day administration of vonoprazan in maintenance treatment of erosive GERD: study protocol for a multicentre randomised cross-over study
- PMID: 29527318
- PMCID: PMC5841500
- DOI: 10.1136/bmjgast-2017-000197
Study for every other day administration of vonoprazan in maintenance treatment of erosive GERD: study protocol for a multicentre randomised cross-over study
Abstract
Introduction: The first drug selected for treatment of gastro-oesophageal reflux disease (GERD) and prevention of the recurrence is a proton pump inhibitor (PPI), but recently, a potassium-competitive acid blocker (P-CAB) was put on the market in Japan. Its onset of effect is faster than PPI, and it takes more than 2 days to recover acid secretion after the withdrawal period. Therefore, unlike PPI, the usefulness of every other day administration or discontinuous administration is expected.
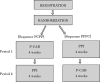
Methods and analysis: This study is a prospective, multicentre, open-label, two-period randomised cross-over study to compare the efficacy and safety of PPI every other day administration and P-CAB every other day administration in 120 patients who receive erosive GERD maintenance therapy with PPI. Patients will be randomly allocated to receive 4 weeks P-CAB or PPI followed by 4 weeks cross over, where those on P-CAB will receive PPI and vice versa. The primary endpoint is proportion of asymptomatic patients. Secondary endpoints are suppressive effect of GERD symptoms, proportion of asymptomatic patients at each time point, safety and cost-saving effect of P-CAB every other day administration, compliance with every other day administration, and proportion of asymptomatic patients at the first month of study drug administration.
Ethics and dissemination: This study was approved by the National Hospital Organization Central Review Board for Clinical Trials (5 December 2017).
Discussion: If P-CAB every other day administration is established as one of GERD maintenance therapies, there is merit in both medical cost reduction and the safety to alleviate elevation in serum gastrin.
Trial registration number: UMIN000034701.
Keywords: acid; gastric acid secretion; gastro-esophageal reflux disease.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Iwakiri K, Kinoshita Y, Habu Y, et al. . Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 2016;51:751–67. doi:10.1007/s00535-016-1227-8 - DOI - PubMed
-
- Jenkins H, Sakurai Y, Nishimura A, et al. . Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015;41:636–48. doi:10.1111/apt.13121 - DOI - PMC - PubMed
-
- Sakurai Y, Mori Y, Okamoto H, et al. . Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther 2015;42:719–30. doi:10.1111/apt.13325 - DOI - PubMed
-
- Sakurai Y, Nishimura A, Kennedy G, et al. . Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015;6:e94 doi:10.1038/ctg.2015.18 - DOI - PMC - PubMed
-
- Savarino E, Martinucci I, Furnari M, et al. . Vonoprazan for treatment of gastroesophageal reflux: pharmacodynamic and pharmacokinetic considerations. Expert Opin Drug Metab Toxicol 2016;8:1333–41. doi:10.1080/17425255.2016.1214714 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources