The dual-inhibitory effect of miR-338-5p on the multidrug resistance and cell growth of hepatocellular carcinoma
- PMID: 29527329
- PMCID: PMC5837112
- DOI: 10.1038/s41392-017-0003-4
The dual-inhibitory effect of miR-338-5p on the multidrug resistance and cell growth of hepatocellular carcinoma
Abstract
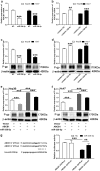
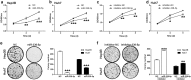
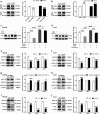
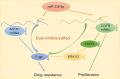
Chemotherapeutic treatments against hepatocellular carcinoma (HCC) are necessary for both inoperable patients to improve prospects for survival and surgery patients to improve the outcome after surgical resection. However, multidrug resistance (MDR) is a major obstacle to obtaining desirable results. Currently, increasing the chemotherapy sensitivity of tumor cells or discovering novel tumor inhibitors is an effective therapeutic strategy to solve this issue. In the present study, we uncovered the dual-inhibitory effect of miR-338-5p: on the one hand, it could downregulate ABCB1 expression and sensitize HCC cells to doxorubicin and vinblastine by directly targeting the 3'-untranslated region (3'-UTR) of ABCB1, while, on the other hand, it could suppress the proliferation of HCC cells by directly targeting the 3'-UTR of EGFR and reducing EGFR expression. Since EGFR regulates ABCB1 levels, the indirect action of miR-338-5p in ABCB1 modulation was revealed, in which miR-338-5p inhibits ABCB1 expression by targeting the EGFR/ERK1/2 signaling pathway. These data indicate that the miR-338-5p/EGFR/ABCB1 regulatory loop plays a critical role in HCC, and a negative correlation between miR-338-5p and EGFR or ABCB1 was also detected in HCC clinical samples. In conclusion, these findings reveal a critical role for miR-338-5p in the regulation of MDR and proliferation of HCC, suggesting the potential therapeutic implications of miR-338-5p in HCC treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
The miR-491-3p/Sp3/ABCB1 axis attenuates multidrug resistance of hepatocellular carcinoma.Cancer Lett. 2017 Nov 1;408:102-111. doi: 10.1016/j.canlet.2017.08.027. Epub 2017 Aug 24. Cancer Lett. 2017. PMID: 28844709
-
miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1.Oncogene. 2014 Jun 19;33(25):3267-76. doi: 10.1038/onc.2013.297. Epub 2013 Jul 29. Oncogene. 2014. PMID: 23893241
-
MicroRNA-548a-5p promotes proliferation and inhibits apoptosis in hepatocellular carcinoma cells by targeting Tg737.World J Gastroenterol. 2016 Jun 21;22(23):5364-73. doi: 10.3748/wjg.v22.i23.5364. World J Gastroenterol. 2016. PMID: 27340352 Free PMC article.
-
MicroRNA-6809-5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1.Phytomedicine. 2019 Apr;57:18-29. doi: 10.1016/j.phymed.2018.10.027. Epub 2018 Oct 22. Phytomedicine. 2019. PMID: 30668319
-
MiR-223 modulates multidrug resistance via downregulation of ABCB1 in hepatocellular carcinoma cells.Exp Biol Med (Maywood). 2013 Sep;238(9):1024-32. doi: 10.1177/1535370213497321. Epub 2013 Aug 7. Exp Biol Med (Maywood). 2013. PMID: 23925649
Cited by
-
Chemical compound cinobufotalin potently induces FOXO1-stimulated cisplatin sensitivity by antagonizing its binding partner MYH9.Signal Transduct Target Ther. 2019 Nov 18;4:48. doi: 10.1038/s41392-019-0084-3. eCollection 2019. Signal Transduct Target Ther. 2019. PMID: 31754475 Free PMC article.
-
Identification of miR-515-3p and its targets, vimentin and MMP3, as a key regulatory mechanism in esophageal cancer metastasis: functional and clinical significance.Signal Transduct Target Ther. 2020 Nov 27;5(1):271. doi: 10.1038/s41392-020-00275-8. Signal Transduct Target Ther. 2020. PMID: 33243974 Free PMC article.
-
Single-Cell RNA Sequencing Integrated with Bulk-RNA Sequencing Analysis Reveals Prognostic Signatures Based on PANoptosis in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2025 Jul 29;12:1661-1676. doi: 10.2147/JHC.S533777. eCollection 2025. J Hepatocell Carcinoma. 2025. PMID: 40761429 Free PMC article.
-
CircularRNA Hsa_circ_0093335 promotes hepatocellular carcinoma progression via sponging miR-338-5p.J Cell Mol Med. 2023 Dec;27(24):4080-4092. doi: 10.1111/jcmm.17991. Epub 2023 Oct 14. J Cell Mol Med. 2023. PMID: 37837352 Free PMC article.
-
Association of a Novel DOCK2 Mutation-Related Gene Signature With Immune in Hepatocellular Carcinoma.Front Genet. 2022 May 10;13:872224. doi: 10.3389/fgene.2022.872224. eCollection 2022. Front Genet. 2022. PMID: 35620462 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

