Predictors of treatment response to liraglutide in type 2 diabetes in a real-world setting
- PMID: 29527621
- PMCID: PMC5959971
- DOI: 10.1007/s00592-018-1124-0
Predictors of treatment response to liraglutide in type 2 diabetes in a real-world setting
Abstract
Aims: There is an unmet need among healthcare providers to identify subgroups of patients with type 2 diabetes who are most likely to respond to treatment.
Methods: Data were taken from electronic medical records of participants of an observational, retrospective study in Italy. We used logistic regression models to assess the odds of achieving glycated haemoglobin (HbA1c) reduction ≥ 1.0% point after 12-month treatment with liraglutide (primary endpoint), according to various patient-related factors. RECursive Partitioning and AMalgamation (RECPAM) analysis was used to identify distinct homogeneous patient subgroups with different odds of achieving the primary endpoint.
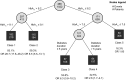
Results: Data from 1325 patients were included, of which 577 (43.5%) achieved HbA1c reduction ≥ 1.0% point (10.9 mmol/mol) after 12 months. Logistic regression showed that for each additional 1% HbA1c at baseline, the odds of reaching this endpoint were increased 3.5 times (95% CI: 2.90-4.32). By use of RECPAM analysis, five distinct responder subgroups were identified, with baseline HbA1c and diabetes duration as the two splitting variables. Patients in the most poorly controlled subgroup (RECPAM Class 1, mean baseline HbA1c > 9.1% [76 mmol/mol]) had a 28-fold higher odds of reaching the endpoint versus patients in the best-controlled group (mean baseline HbA1c ≤ 7.5% [58 mmol/mol]). Mean HbA1c reduction from baseline was as large as - 2.2% (24 mol/mol) in the former versus - 0.1% (1.1 mmol/mol) in the latter. Mean weight reduction ranged from 2.5 to 4.3 kg across RECPAM subgroups.
Conclusions: Glycaemic response to liraglutide is largely driven by baseline HbA1c levels and, to a lesser extent, by diabetes duration.
Keywords: GLP-1RA; Liraglutide; RECPAM analysis; Response to therapy; Type 2 diabetes.
Conflict of interest statement
Conflict of interest
Simioni N: Consulting fees from Novo Nordisk, Lilly, Boehringer Ingelheim and Abbott; member of advisory boards for Novo Nordisk, Lilly and Boehringer Ingelheim; investigator in clinical trials sponsored by Novo Nordisk. Berra C: consulting fees from Novo Nordisk, Lilly, Boehringer Ingelheim, Sanofi, Johnson & Johnson and Bayer; research support from AstraZeneca and Takeda; member of advisory boards for Novo Nordisk, Lilly, Boehringer Ingelheim, AstraZeneca and Sanofi; investigator in clinical trials sponsored by Lilly and Sanofi. Boemi M: member of advisory boards for Lilly, Boehringer Ingelheim and Sanofi; investigator in clinical trials sponsored by Novo Nordisk, Boehringer Ingelheim and Merck SD. Bossi AC: investigator in clinical trials sponsored by Novo Nordisk, Artsana, Lilly, Bayer and Sanofi; consulting fees from AstraZeneca, Roche, Johnson & Johnson and Takeda; research support from Merck SD and Sigma-Tau; member of advisory board for Boehringer Ingelheim. Candido R: investigator in clinical trials sponsored by Novo Nordisk, Lilly and Merck SD; consulting fees from AstraZeneca, Roche and Johnson & Johnson; member of advisory board for Boehringer Ingelheim. Di Cianni G: investigator in clinical trials sponsored by Novo Nordisk, AstraZeneca and Sanofi; member of advisory boards for Lilly and Sanofi. Frontoni S: member of advisory boards for Novo Nordisk, Lilly, AstraZeneca, Johnson & Johnson, Takeda and Sigma-Tau; investigator in clinical trials sponsored by Novo Nordisk and Boehringer Ingelheim. Genovese S: consulting fees from Novo Nordisk, Lilly, Boehringer Ingelheim, AstraZeneca, Merck SD, Sanofi, Johnson & Johnson, Takeda, Abbott Diabetes Care, Bristol Myers & Squibb, Janssen, Lifescan, Menarini and Novartis; member of advisory boards for Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Merck SD, Sanofi, Johnson & Johnson, Takeda, Abbott Diabetes Care, Bruno Farmaceutici, Janssen, Lifescan and Novartis; research support from Novartis; investigator in clinical trials sponsored by Novo Nordisk, Lilly, Boehringer Ingelheim, AstraZeneca, Merck SD, Takeda, Janssen, Novartis and Sanofi. Ponzani P: investigator in clinical trials sponsored by Boehringer Ingelheim, Sanofi, Johnson & Johnson, Bayer and Novartis; member of advisory boards for Novo Nordisk and AstraZeneca. Provenzano V: consulting fees from Novo Nordisk, Lilly, Boehringer Ingelheim, AstraZeneca, Merck SD, Sanofi and Takeda; member of advisory boards for Novo Nordisk, Lilly, Boehringer Ingelheim, AstraZeneca and Sanofi; investigator in clinical trials sponsored by Novo Nordisk, Lilly, Boehringer Ingelheim, AstraZeneca, Merck SD, Sanofi and Roche. Russo GT: investigator in clinical trials sponsored by Lilly, Boehringer Ingelheim, Merck SD, Sanofi and Johnson & Johnson; member of advisory boards for Novo Nordisk, Lilly and Boehringer Ingelheim, member of advisory boards for, and consulting fees from, Novo Nordisk, Lilly and Boehringer Ingelheim. Sciangula L: member of advisory boards for Novo Nordisk, Lilly, AstraZeneca and Johnson & Johnson; consulting fees from Roche; investigator in clinical trials sponsored by Novo Nordisk. Lapolla A: investigator in clinical trials sponsored by Novo Nordisk, Lilly, Boehringer Ingelheim and Sanofi. Bette C: employee of Novo Nordisk SpA (Rome, Italy). Rossi MC: research grant from Novo Nordisk, Sanofi, Dexcom, AstraZeneca, Sigma-Tau, Eli Lilly, Artsana and Medtronic.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practices. According to Italian law (Italian Republic. Determination of the Italian Medicines Agency of March 20, 2008. Official Gazette of the Italian Republic. General Series No. 76; March 31, 2008), prior to study initiation, the protocol, patient informed consent form and patient enrolment procedures were reviewed and approved by an Independent Ethics Committee (IEC). The study protocol was submitted to the Coordinating Centre IEC in advance, then after its official approval, the study documentation was submitted to the local IECs of all participating centres.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Victoza® summary of product characteristics (2016)
-
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. - DOI - PMC - PubMed
-
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;3:84–90. doi: 10.2337/dc08-1355. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

