GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels
- PMID: 29527745
- PMCID: PMC6135720
- DOI: 10.1111/xen.12391
GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels
Abstract
Background: Despite recent progress in survival times of xenografts in non-human primates, there are no reports of survival beyond 5 days of histologically well-aerated porcine lung grafts in baboons. Here, we report our initial results of pig-to-baboon xeno-lung transplantation (XLTx).
Methods: Eleven baboons received genetically modified porcine left lungs from either GalT-KO alone (n = 3), GalT-KO/humanCD47(hCD47)/hCD55 (n = 3), GalT-KO/hD47/hCD46 (n = 4), or GalT-KO/hCD39/hCD46/hCD55/TBM/EPCR (n = 1) swine. The first 2 XLTx procedures were performed under a non-survival protocol that allowed a 72-hour follow-up of the recipients with general anesthesia, while the remaining 9 underwent a survival protocol with the intention of weaning from ventilation.
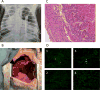
Results: Lung graft survivals in the 2 non-survival animals were 48 and >72 hours, while survivals in the other 9 were 25 and 28 hours, at 5, 5, 6, 7, >7, 9, and 10 days. One baboon with graft survival >7 days, whose entire lung graft remained well aerated, was euthanized on POD 7 due to malfunction of femoral catheters. hCD47 expression of donor lungs was detected in both alveoli and vessels only in the 3 grafts surviving >7, 9, and 10 days. All other grafts lacked hCD47 expression in endothelial cells and were completely rejected with diffuse hemorrhagic changes and antibody/complement deposition detected in association with early graft loss.
Conclusions: To our knowledge, this is the first evidence of histologically viable porcine lung grafts beyond 7 days in baboons. Our results indicate that GalT-KO pig lungs are highly susceptible to acute humoral rejection and that this may be mitigated by transgenic expression of hCD47.
Keywords: hCD47; lung transplantation; pig to baboon; xenotransplantation.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures











Similar articles
-
Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation.Xenotransplantation. 2021 Nov;28(6):e12708. doi: 10.1111/xen.12708. Epub 2021 Aug 21. Xenotransplantation. 2021. PMID: 34418164 Free PMC article.
-
Antibody reactivity with new antigens revealed in multi-transgenic triple knockout pigs may cause early loss of pig kidneys in baboons.Xenotransplantation. 2021 Jan;28(1):e12642. doi: 10.1111/xen.12642. Epub 2020 Sep 9. Xenotransplantation. 2021. PMID: 32909301 Free PMC article.
-
Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival.Xenotransplantation. 2020 Jan;27(1):e12552. doi: 10.1111/xen.12552. Epub 2019 Sep 23. Xenotransplantation. 2020. PMID: 31544995 Free PMC article.
-
Lung xenotransplantation.Curr Opin Organ Transplant. 2017 Dec;22(6):541-548. doi: 10.1097/MOT.0000000000000465. Curr Opin Organ Transplant. 2017. PMID: 28872471 Free PMC article. Review.
-
Progress and challenges in lung xenotransplantation: an update.Curr Opin Organ Transplant. 2018 Dec;23(6):621-627. doi: 10.1097/MOT.0000000000000582. Curr Opin Organ Transplant. 2018. PMID: 30234737 Review.
Cited by
-
Preparation of hybrid porcine thymus containing non-human primate thymic epithelial cells in miniature swine.Xenotransplantation. 2019 Nov;26(6):e12543. doi: 10.1111/xen.12543. Epub 2019 Jul 10. Xenotransplantation. 2019. PMID: 31293016 Free PMC article.
-
Profiling Human CD55 Transgene Performance Assist in Selecting Best Suited Specimens and Tissues for Swine Organ Xenotransplantation.Biology (Basel). 2021 Aug 4;10(8):747. doi: 10.3390/biology10080747. Biology (Basel). 2021. PMID: 34439979 Free PMC article.
-
Genetic engineering of pigs for xenotransplantation to overcome immune rejection and physiological incompatibilities: The first clinical steps.Front Immunol. 2022 Dec 6;13:1031185. doi: 10.3389/fimmu.2022.1031185. eCollection 2022. Front Immunol. 2022. PMID: 36561750 Free PMC article. Review.
-
Future of Lung Transplantation: Xenotransplantation and Bioengineering Lungs.Clin Chest Med. 2023 Mar;44(1):201-214. doi: 10.1016/j.ccm.2022.11.003. Clin Chest Med. 2023. PMID: 36774165 Free PMC article. Review.
-
Efficient generation of GGTA1-deficient pigs by electroporation of the CRISPR/Cas9 system into in vitro-fertilized zygotes.BMC Biotechnol. 2020 Aug 18;20(1):40. doi: 10.1186/s12896-020-00638-7. BMC Biotechnol. 2020. PMID: 32811500 Free PMC article.
References
-
- Klassen DK, Edwards LB, Stewart DE, Glazier AK, Orlowski JP, Berg CL. The OPTN Deceased Donor Potential Study: Implications for Policy and Practice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(6):1707–1714. doi: 10.1111/ajt.13731. - DOI - PubMed
-
- Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report–2015; Focus Theme: Early Graft Failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(10):1264–1277. doi: 10.1016/j.healun.2015.08.014. - DOI - PubMed
-
- Sachs DH. The pig as a potential xenograft donor. Veterinary immunology and immunopathology. 1994;43(1–3):185–191. - PubMed
-
- Sato M, Kagoshima A, Saitoh I, Inada E, Miyoshi K, Ohtsuka M, et al. Generation of alpha-1,3-Galactosyltransferase-Deficient Porcine Embryonic Fibroblasts by CRISPR/Cas9-Mediated Knock-in of a Small Mutated Sequence and a Targeted Toxin-Based Selection System. Reprod Domest Anim. 2015;50(5):872–880. doi: 10.1111/rda.12565. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

