Expression of C9orf72-related dipeptides impairs motor function in a vertebrate model
- PMID: 29528390
- PMCID: PMC5932562
- DOI: 10.1093/hmg/ddy083
Expression of C9orf72-related dipeptides impairs motor function in a vertebrate model
Abstract
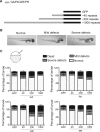
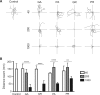
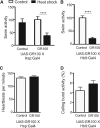
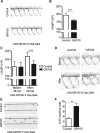
Large expansions of hexanucleotide GGGGCC (G4C2) repeats (hundreds to thousands) in the first intron of the chromosome 9 open reading frame 72 (C9orf72) locus are the strongest known genetic factor associated with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Different hypotheses exist about the underlying disease mechanism including loss of function by haploinsufficiency, toxicity arising as a result of RNA or dipeptide repeats (DPRs). Five different DPRs are produced by repeat-associated non-ATG-initiated translation of the G4C2 repeats. Though earlier studies have indicated toxicity of the DPRs in worms, flies, primary cultured cells and cell lines, the effect of expressing DPRs of amyotrophic lateral sclerosis-relevant length has not been tested on motor behaviour in vertebrate models. In this study, by expressing constructs with alternate codons encoding different lengths of each DPR (40, 200 and 1000) in the vertebrate zebrafish model, the GR DPR was found to lead to the greatest developmental lethality and morphological defects, and GA, the least. However, expressing 1000 repeats of any DPR, including the 'non-toxic' GA DPR led to locomotor defects. Based on these observations, a transgenic line stably expressing 100 GR repeats was generated to allow specific regional and temporal expression of GR repeats in vivo. Expression of GR DPRs ubiquitously resulted in severe morphological defects and reduced swimming. However, when expressed specifically in motor neurons, the developmental defects were significantly reduced, but the swimming phenotype persisted, suggesting that GR DPRs have a toxic effect on motor neuron function. This was validated by the reduction in motor neuron length even in already formed motor neurons when GR was expressed in these. Hence, the expression of C9orf72-associated DPRs can cause significant motor deficits in vertebrates.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

