Naltrexone maintenance fails to alter amphetamine effects on intracranial self-stimulation in rats
- PMID: 29528663
- PMCID: PMC5897164
- DOI: 10.1037/pha0000183
Naltrexone maintenance fails to alter amphetamine effects on intracranial self-stimulation in rats
Abstract
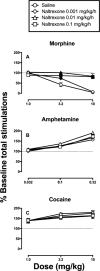
Pharmacotherapy to treat stimulant use disorders continues to be an unmet medical need. Some evidence supports both the role of opioids in mediating abuse-related amphetamine effects and the potential utility of opioid antagonists as therapeutic candidates for treating amphetamine abuse. This study used intracranial self-stimulation (ICSS) to evaluate effects of exposure to and termination of naltrexone maintenance on rewarding amphetamine effects in an ICSS procedure in rats. Morphine and cocaine were included as positive and negative controls, respectively. Male Sprague-Dawley rats (N = 40) were trained to lever press for electrical brain stimulation to the medial forebrain bundle via an implanted electrode. Rats were then implanted with osmotic pumps delivering naltrexone (0.001 mg/kg/h, SC, 0.01 mg/kg/h, SC, or 0.1 mg/kg/h, SC) or saline for 14 days. Cumulative dose-effect curves were determined for amphetamine (0.032 mg/kg to 0.32 mg/kg), cocaine (1 mg/kg to 10 mg/kg), and morphine (1 mg/kg to 10 mg/kg) during the 2nd week of naltrexone maintenance. Additionally, dose-effect curves for morphine and amphetamine were determined again 24 hr after pump removal. Our results suggest that (a) exposure to and termination of naltrexone maintenance do not affect baseline ICSS responding, (b) naltrexone doses sufficient to antagonize morphine did not alter amphetamine or cocaine effects, and (c) termination of naltrexone treatment produced weak evidence for increased morphine sensitivity but no change in amphetamine effects. Our results do not support naltrexone as a pharmacotherapy for amphetamine and cocaine abuse and also suggest that termination from chronic naltrexone does not increase sensitivity to abuse-related morphine or amphetamine effects in ICSS. (PsycINFO Database Record
(c) 2018 APA, all rights reserved).
Conflict of interest statement
The authors have no conflict to declare.
Figures



Similar articles
-
Determinants of opioid abuse potential: Insights using intracranial self-stimulation.Peptides. 2019 Feb;112:23-31. doi: 10.1016/j.peptides.2018.10.007. Epub 2018 Nov 1. Peptides. 2019. PMID: 30391425 Free PMC article. Review.
-
Abuse-related effects of µ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure.Behav Pharmacol. 2013 Sep;24(5-6):459-70. doi: 10.1097/FBP.0b013e328364c0bd. Behav Pharmacol. 2013. PMID: 23881045 Free PMC article.
-
The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats.Psychopharmacology (Berl). 2014 Jun;231(12):2461-70. doi: 10.1007/s00213-013-3405-1. Epub 2014 Jan 10. Psychopharmacology (Berl). 2014. PMID: 24408209 Free PMC article.
-
Preclinical Abuse Potential Assessment of Flibanserin: Effects on Intracranial Self-Stimulation in Female and Male Rats.J Sex Med. 2016 Mar;13(3):338-49. doi: 10.1016/j.jsxm.2015.12.031. Epub 2016 Jan 28. J Sex Med. 2016. PMID: 26831817 Free PMC article.
-
Drug-induced neurobehavioral plasticity: the role of environmental context.Behav Pharmacol. 2004 Sep;15(5-6):327-39. doi: 10.1097/00008877-200409000-00004. Behav Pharmacol. 2004. PMID: 15343056 Review.
Cited by
-
Determinants of opioid abuse potential: Insights using intracranial self-stimulation.Peptides. 2019 Feb;112:23-31. doi: 10.1016/j.peptides.2018.10.007. Epub 2018 Nov 1. Peptides. 2019. PMID: 30391425 Free PMC article. Review.
-
Effectiveness and selectivity of a heroin conjugate vaccine to attenuate heroin, 6-acetylmorphine, and morphine antinociception in rats: Comparison with naltrexone.Drug Alcohol Depend. 2019 Nov 1;204:107501. doi: 10.1016/j.drugalcdep.2019.06.006. Epub 2019 Aug 24. Drug Alcohol Depend. 2019. PMID: 31479865 Free PMC article.
-
Effects of acute and repeated treatment with serotonin 5-HT2A receptor agonist hallucinogens on intracranial self-stimulation in rats.Exp Clin Psychopharmacol. 2019 Jun;27(3):215-226. doi: 10.1037/pha0000253. Epub 2019 Jan 10. Exp Clin Psychopharmacol. 2019. PMID: 30628811 Free PMC article.
-
Effects of naltrexone on amphetamine choice in rhesus monkeys and rats.Exp Clin Psychopharmacol. 2023 Dec;31(6):1080-1091. doi: 10.1037/pha0000655. Epub 2023 May 15. Exp Clin Psychopharmacol. 2023. PMID: 37184942 Free PMC article.
-
Interactions between Cocaine and the Putative Allosteric Dopamine Transporter Ligand SRI-31142.J Pharmacol Exp Ther. 2018 Nov;367(2):222-233. doi: 10.1124/jpet.118.250902. Epub 2018 Aug 27. J Pharmacol Exp Ther. 2018. PMID: 30150482 Free PMC article.
References
-
- Balcells-Olivero M, Vezina P. Effects of naltrexone on amphetamine-induced locomotion and rearing: acute and repeated injections. Psychopharmacology. 1997;131(3):230–238. - PubMed
-
- Bardo MT, Neisewander JL, Ennis RB. Chronic treatment with naltrexone enhances morphine-stimulated dopamine neurotransmission: neurochemical and behavioral evidence. Neuropharmacology. 1988;27(11):1103–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

