Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons
- PMID: 29528964
- PMCID: PMC5959002
- DOI: 10.1097/j.pain.0000000000001201
Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons
Abstract
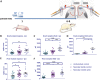
Noxious stimulation at critical stages of development has long-term consequences on somatosensory processing in later life, but it is not known whether this developmental plasticity is restricted to nociceptive pathways. Here, we investigate the effect of repeated neonatal noxious or innocuous hind paw stimulation on adult spinal dorsal horn cutaneous mechanical sensitivity. Neonatal Sprague-Dawley rats of both sexes received 4 unilateral left hind paw needle pricks (NPs, n = 13) or 4 tactile (cotton swab touch) stimuli, per day (TC, n = 11) for the first 7 days of life. Control pups were left undisturbed (n = 17). When adult (6-8 weeks), lumbar wide-dynamic-range neuron activity in laminae III-V was recorded using in vivo extracellular single-unit electrophysiology. Spike activity evoked by cutaneous dynamic tactile (brush), pinch and punctate (von Frey hair) stimulation, and plantar receptive field areas were recorded, at baseline and 2 and 5 days after left plantar hind paw incision. Baseline brush receptive fields, von Frey hair, and pinch sensitivity were significantly enhanced in adult NP and TC animals compared with undisturbed controls, although effects were greatest in NP rats. After incision, injury sensitivity of adult wide-dynamic-range neurons to both noxious and dynamic tactile hypersensitivity was significantly greater in NP animals compared with TC and undisturbed controls. We conclude that both repeated touch and needle-prick stimulation in the neonatal period can alter adult spinal sensory neuron sensitivity to both innocuous and noxious mechanical stimulation. Thus, spinal sensory circuits underlying touch and pain processing are shaped by a range of early-life somatosensory experiences.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



References
-
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O'Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 2017;168:295–310.e219. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

