Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization
- PMID: 29529016
- PMCID: PMC5893385
- DOI: 10.1038/nm.4491
Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization
Abstract
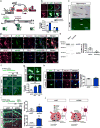
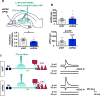
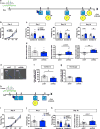
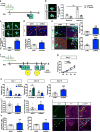
Memories become less precise and generalized over time as memory traces reorganize in hippocampal-cortical networks. Increased time-dependent loss of memory precision is characterized by an overgeneralization of fear in individuals with post-traumatic stress disorder (PTSD) or age-related cognitive impairments. In the hippocampal dentate gyrus (DG), memories are thought to be encoded by so-called 'engram-bearing' dentate granule cells (eDGCs). Here we show, using rodents, that contextual fear conditioning increases connectivity between eDGCs and inhibitory interneurons (INs) in the downstream hippocampal CA3 region. We identify actin-binding LIM protein 3 (ABLIM3) as a mossy-fiber-terminal-localized cytoskeletal factor whose levels decrease after learning. Downregulation of ABLIM3 expression in DGCs was sufficient to increase connectivity with CA3 stratum lucidum INs (SLINs), promote parvalbumin (PV)-expressing SLIN activation, enhance feedforward inhibition onto CA3 and maintain a fear memory engram in the DG over time. Furthermore, downregulation of ABLIM3 expression in DGCs conferred conditioned context-specific reactivation of memory traces in hippocampal-cortical and amygdalar networks and decreased fear memory generalization at remote (i.e., distal) time points. Consistent with the observation of age-related hyperactivity of CA3, learning failed to increase DGC-SLIN connectivity in 17-month-old mice, whereas downregulation of ABLIM3 expression was sufficient to restore DGC-SLIN connectivity, increase PV+ SLIN activation and improve the precision of remote memories. These studies exemplify a connectivity-based strategy that targets a molecular brake of feedforward inhibition in DG-CA3 and may be harnessed to decrease time-dependent memory generalization in individuals with PTSD and improve memory precision in aging individuals.
Conflict of interest statement
The authors declare competing financial interests. A.S and N.G are named co-inventors on patent application relating to this study.
Figures


Comment in
-
A molecular mechanism governing memory precision.Nat Med. 2018 Apr;24(4):390-391. doi: 10.1038/nm.4532. Nat Med. 2018. PMID: 29634686 No abstract available.
References
Supplementary References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

