Localization of RNA and translation in the mammalian oocyte and embryo
- PMID: 29529035
- PMCID: PMC5846722
- DOI: 10.1371/journal.pone.0192544
Localization of RNA and translation in the mammalian oocyte and embryo
Abstract
The tight correlation between mRNA distribution and subsequent protein localization and function indicate a major role for mRNA localization within the cell. RNA localization, followed by local translation, presents a mechanism for spatial and temporal gene expression regulation utilized by various cell types. However, little is known about mRNA localization and translation in the mammalian oocyte and early embryo. Importantly, fully-grown oocyte becomes transcriptionally inactive and only utilizes transcripts previously synthesized and stored during earlier development. We discovered an abundant RNA population in the oocyte and early embryo nucleus together with RNA binding proteins. We also characterized specific ribosomal proteins, which contribute to translation in the oocyte and embryo. By applying selected markers to mouse and human oocytes, we found that there might be a similar mechanism of RNA metabolism in both species. In conclusion, we visualized the localization of RNAs and translation machinery in the oocyte, that could shed light on this terra incognita of these unique cell types in mouse and human.
Conflict of interest statement
Figures

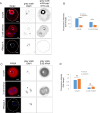
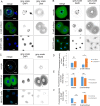

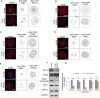
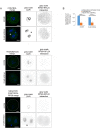


References
-
- Schultz RM. Regulation of zygotic gene activation in the mouse. BioEssays News Rev Mol Cell Dev Biol. 1993;15: 531–538. doi: 10.1002/bies.950150806 - DOI - PubMed
-
- Ellederova Z, Kovarova H, Melo-Sterza F, Livingstone M, Tomek W, Kubelka M. Suppression of translation during in vitro maturation of pig oocytes despite enhanced formation of cap-binding protein complex eIF4F and 4E-BP1 hyperphosphorylation. Mol Reprod Dev. 2006;73: 68–76. doi: 10.1002/mrd.20368 - DOI - PubMed
-
- Tomek W, Melo Sterza FA, Kubelka M, Wollenhaupt K, Torner H, Anger M, et al. Regulation of translation during in vitro maturation of bovine oocytes: the role of MAP kinase, eIF4E (cap binding protein) phosphorylation, and eIF4E-BP1. Biol Reprod. 2002;66: 1274–1282. - PubMed
-
- Flemr M, Ma J, Schultz RM, Svoboda P. P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol Reprod. 2010;82: 1008–1017. doi: 10.1095/biolreprod.109.082057 - DOI - PMC - PubMed
-
- Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280: 585–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

