Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol
- PMID: 29529169
- PMCID: PMC5961425
- DOI: 10.1093/annonc/mdy072
Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol
Abstract
Background: Adding abiraterone acetate with prednisolone (AAP) or docetaxel with prednisolone (DocP) to standard-of-care (SOC) each improved survival in systemic therapy for advanced or metastatic prostate cancer: evaluation of drug efficacy: a multi-arm multi-stage platform randomised controlled protocol recruiting patients with high-risk locally advanced or metastatic PCa starting long-term androgen deprivation therapy (ADT). The protocol provides the only direct, randomised comparative data of SOC + AAP versus SOC + DocP.
Method: Recruitment to SOC + DocP and SOC + AAP overlapped November 2011 to March 2013. SOC was long-term ADT or, for most non-metastatic cases, ADT for ≥2 years and RT to the primary tumour. Stratified randomisation allocated pts 2 : 1 : 2 to SOC; SOC + docetaxel 75 mg/m2 3-weekly×6 + prednisolone 10 mg daily; or SOC + abiraterone acetate 1000 mg + prednisolone 5 mg daily. AAP duration depended on stage and intent to give radical RT. The primary outcome measure was death from any cause. Analyses used Cox proportional hazards and flexible parametric models, adjusted for stratification factors. This was not a formally powered comparison. A hazard ratio (HR) <1 favours SOC + AAP, and HR > 1 favours SOC + DocP.
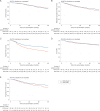
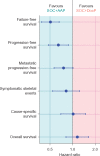
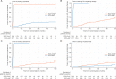
Results: A total of 566 consenting patients were contemporaneously randomised: 189 SOC + DocP and 377 SOC + AAP. The patients, balanced by allocated treatment were: 342 (60%) M1; 429 (76%) Gleason 8-10; 449 (79%) WHO performance status 0; median age 66 years and median PSA 56 ng/ml. With median follow-up 4 years, 149 deaths were reported. For overall survival, HR = 1.16 (95% CI 0.82-1.65); failure-free survival HR = 0.51 (95% CI 0.39-0.67); progression-free survival HR = 0.65 (95% CI 0.48-0.88); metastasis-free survival HR = 0.77 (95% CI 0.57-1.03); prostate cancer-specific survival HR = 1.02 (0.70-1.49); and symptomatic skeletal events HR = 0.83 (95% CI 0.55-1.25). In the safety population, the proportion reporting ≥1 grade 3, 4 or 5 adverse events ever was 36%, 13% and 1% SOC + DocP, and 40%, 7% and 1% SOC + AAP; prevalence 11% at 1 and 2 years on both arms. Relapse treatment patterns varied by arm.
Conclusions: This direct, randomised comparative analysis of two new treatment standards for hormone-naïve prostate cancer showed no evidence of a difference in overall or prostate cancer-specific survival, nor in other important outcomes such as symptomatic skeletal events. Worst toxicity grade over entire time on trial was similar but comprised different toxicities in line with the known properties of the drugs.
Trial registration: Clinicaltrials.gov: NCT00268476.
Figures



Comment in
-
The case for 'successfully' treating hormone naïve metastatic prostate cancer.Ann Oncol. 2018 May 1;29(5):1084-1086. doi: 10.1093/annonc/mdy122. Ann Oncol. 2018. PMID: 29688267 No abstract available.
References
-
- Widmark A, Klepp O, Solberg A. et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373(9660): 301–308. - PubMed
-
- Mottet N, Peneau M, Mazeron JJ. et al. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur Urol 2012; 62(2): 213–219. - PubMed
-
- Fizazi K, Lesaunier F, Delva R. et al. A phase III trial of docetaxel–estramustine in high-risk localised prostate cancer: a planned analysis of response, toxicity and quality of life in the GETUG 12 trial. Eur J Cancer 2012; 48(2): 209–217. - PubMed
-
- Fizazi K, Faivre L, Lesaunier F. et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol 2015; 16(7): 787–794. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

