Differentiating Pathway-Specific From Nonspecific Effects in High-Throughput Toxicity Data: A Foundation for Prioritizing Adverse Outcome Pathway Development
- PMID: 29529260
- PMCID: PMC6820004
- DOI: 10.1093/toxsci/kfy049
Differentiating Pathway-Specific From Nonspecific Effects in High-Throughput Toxicity Data: A Foundation for Prioritizing Adverse Outcome Pathway Development
Abstract
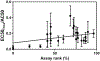
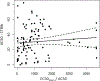
The U.S. Environmental Protection Agency's ToxCast program has screened thousands of chemicals for biological activity, primarily using high-throughput in vitro bioassays. Adverse outcome pathways (AOPs) offer a means to link pathway-specific biological activities with potential apical effects relevant to risk assessors. Thus, efforts are underway to develop AOPs relevant to pathway-specific perturbations detected in ToxCast assays. Previous work identified a "cytotoxic burst" (CTB) phenomenon wherein large numbers of the ToxCast assays begin to respond at or near test chemical concentrations that elicit cytotoxicity, and a statistical approach to defining the bounds of the CTB was developed. To focus AOP development on the molecular targets corresponding to ToxCast assays indicating pathway-specific effects, we conducted a meta-analysis to identify which assays most frequently respond at concentrations below the CTB. A preliminary list of potentially important, target-specific assays was determined by ranking assays by the fraction of chemical hits below the CTB compared with the number of chemicals tested. Additional priority assays were identified using a diagnostic-odds-ratio approach which gives greater ranking to assays with high specificity but low responsivity. Combined, the two prioritization methods identified several novel targets (e.g., peripheral benzodiazepine and progesterone receptors) to prioritize for AOP development, and affirmed the importance of a number of existing AOPs aligned with ToxCast targets (e.g., thyroperoxidase, estrogen receptor, aromatase). The prioritization approaches did not appear to be influenced by inter-assay differences in chemical bioavailability. Furthermore, the outcomes were robust based on a variety of different parameters used to define the CTB.
Figures



References
-
- Ankley GT, Bennet RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder, et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem 29, 730–741. - PubMed
-
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, and Mattson MP (1995). Tumor necrosis factors alpha and beta protect neurons against amyloid betapeptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc. Natl. Acad. Sci. U. S. A 92, 9328–9332. - PMC - PubMed
-
- Barron M, Lilavois C, and Martin T (2015). MOAtox: a comprehensive mode of action and acute aquatic toxicity database for predictive model development. Aquat. Toxicol 161, 102–107. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

