The Microbiome Regulates Pulmonary Responses to Ozone in Mice
- PMID: 29529379
- PMCID: PMC6189641
- DOI: 10.1165/rcmb.2017-0404OC
The Microbiome Regulates Pulmonary Responses to Ozone in Mice
Abstract
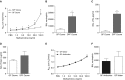
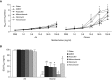
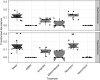
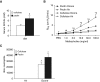
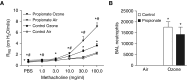
Previous reports demonstrate that the microbiome impacts allergic airway responses, including airway hyperresponsiveness, a characteristic feature of asthma. Here we examined the role of the microbiome in pulmonary responses to a nonallergic asthma trigger, ozone. We depleted the microbiota of conventional mice with either a single antibiotic (ampicillin, metronidazole, neomycin, or vancomycin) or a cocktail of all four antibiotics given via the drinking water. Mice were then exposed to room air or ozone. In air-exposed mice, airway responsiveness did not differ between antibiotic- and control water-treated mice. Ozone caused airway hyperresponsiveness, the magnitude of which was decreased in antibiotic cocktail-treated mice versus water-treated mice. Except for neomycin, single antibiotics had effects similar to those observed with the cocktail. Compared with conventional mice, germ-free mice also had attenuated airway responsiveness after ozone. 16S ribosomal RNA gene sequencing of fecal DNA to characterize the gut microbiome indicated that bacterial genera that were decreased in mice with reduced ozone-induced airway hyperresponsiveness after antibiotic treatment were short-chain fatty acid producers. Serum analysis indicated reduced concentrations of the short-chain fatty acid propionate in cocktail-treated mice but not in neomycin-treated mice. Dietary enrichment with pectin, which increased serum short-chain fatty acids, also augmented ozone-induced airway hyperresponsiveness. Furthermore, propionate supplementation of the drinking water augmented ozone-induced airway hyperresponsiveness in conventional mice. Our data indicate that the microbiome contributes to ozone-induced airway hyperresponsiveness, likely via its ability to produce short-chain fatty acids.
Keywords: 16S rRNA gene sequencing; airway responsiveness; antibiotics; germ-free mice; neutrophil.
Figures

Comment in
-
The Gut-Lung Axis and Pulmonary Responses to Ozone.Am J Respir Cell Mol Biol. 2018 Sep;59(3):281-282. doi: 10.1165/rcmb.2018-0140ED. Am J Respir Cell Mol Biol. 2018. PMID: 29723038 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

