Dissecting the pathobiology of altered MRI signal in amyotrophic lateral sclerosis: A post mortem whole brain sampling strategy for the integration of ultra-high-field MRI and quantitative neuropathology
- PMID: 29529995
- PMCID: PMC5848544
- DOI: 10.1186/s12868-018-0416-1
Dissecting the pathobiology of altered MRI signal in amyotrophic lateral sclerosis: A post mortem whole brain sampling strategy for the integration of ultra-high-field MRI and quantitative neuropathology
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a clinically and histopathologically heterogeneous neurodegenerative disorder, in which therapy is hindered by the rapid progression of disease and lack of biomarkers. Magnetic resonance imaging (MRI) has demonstrated its potential for detecting the pathological signature and tracking disease progression in ALS. However, the microstructural and molecular pathological substrate is poorly understood and generally defined histologically. One route to understanding and validating the pathophysiological correlates of MRI signal changes in ALS is to directly compare MRI to histology in post mortem human brains.
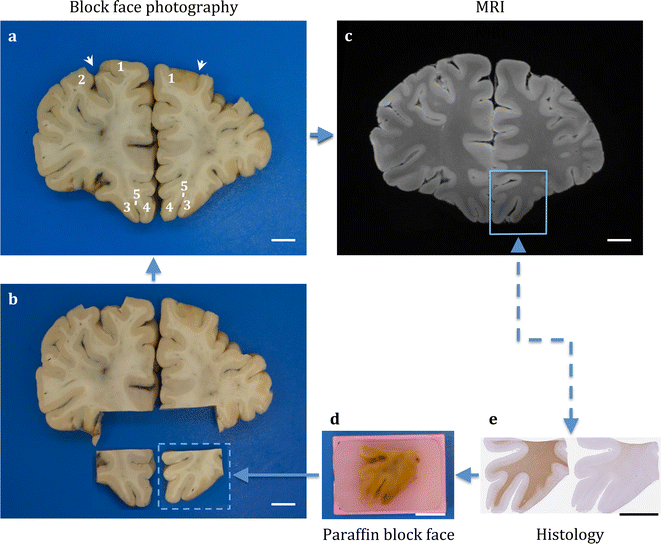
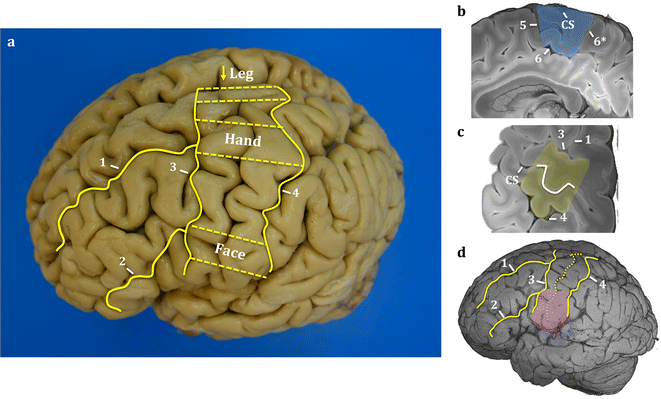
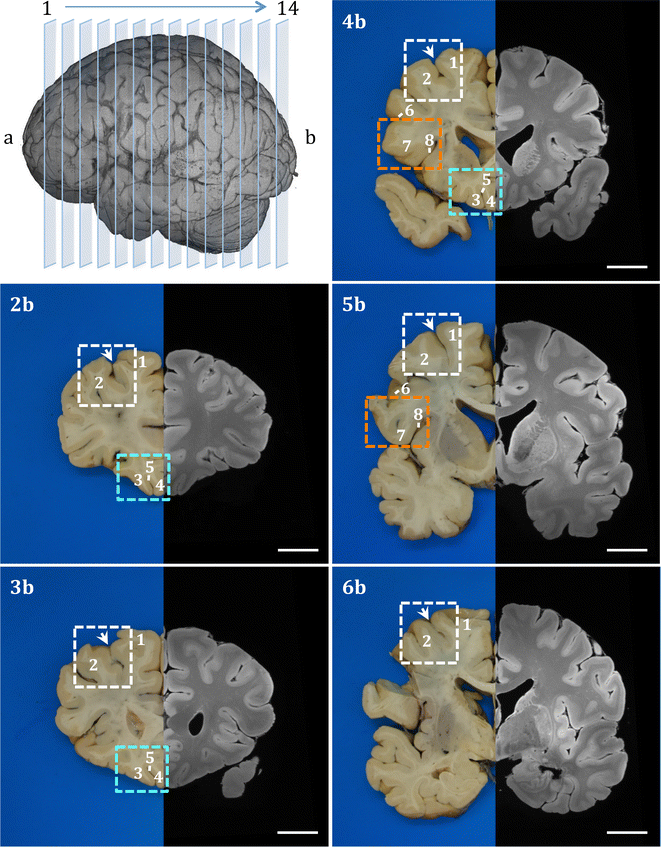
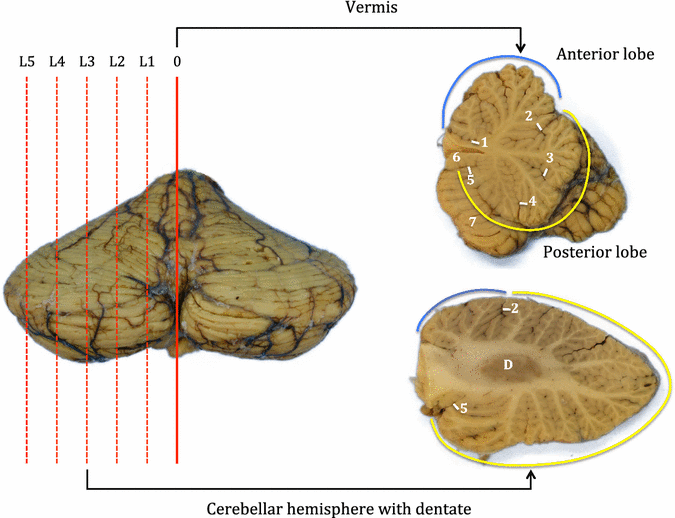
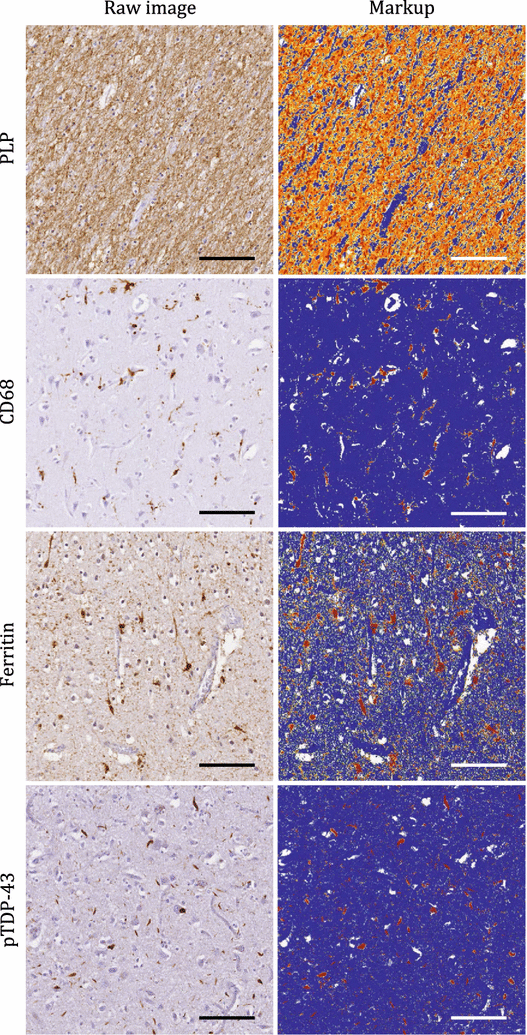
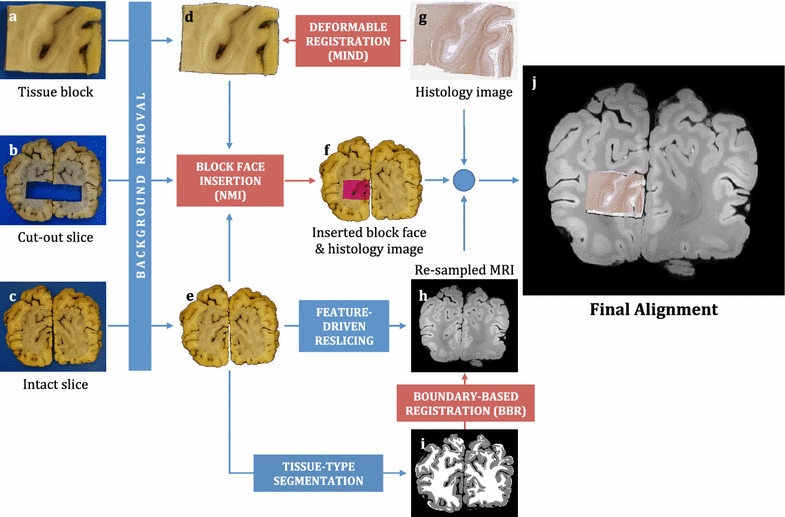
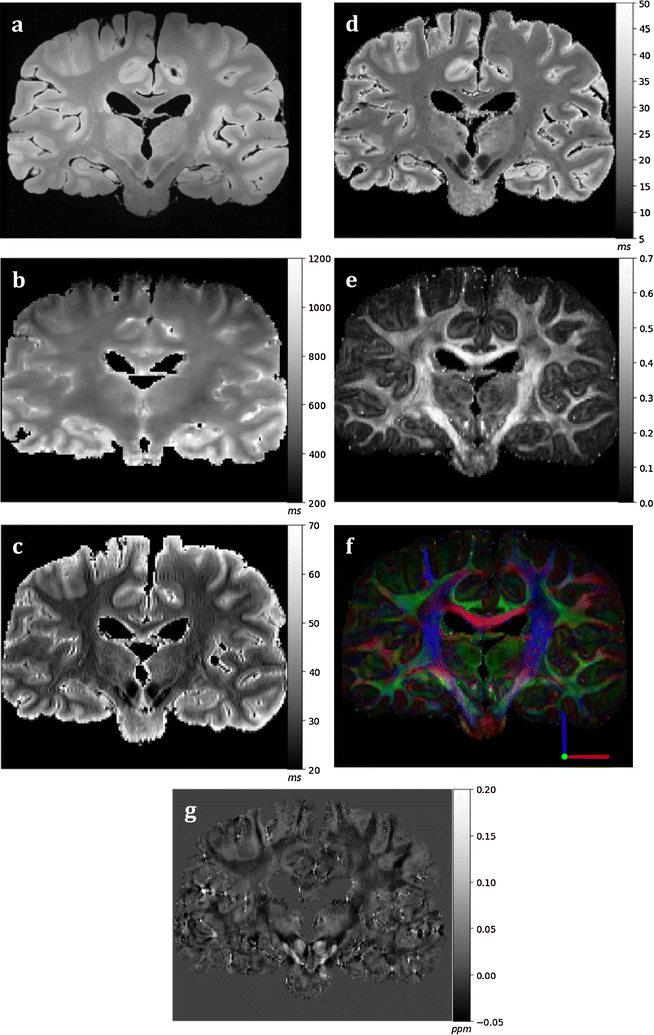
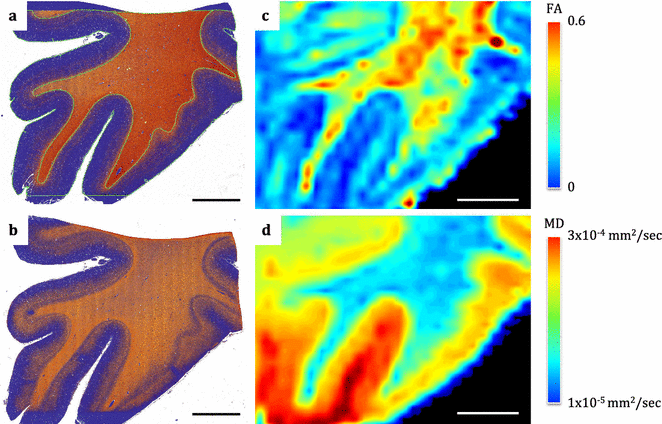
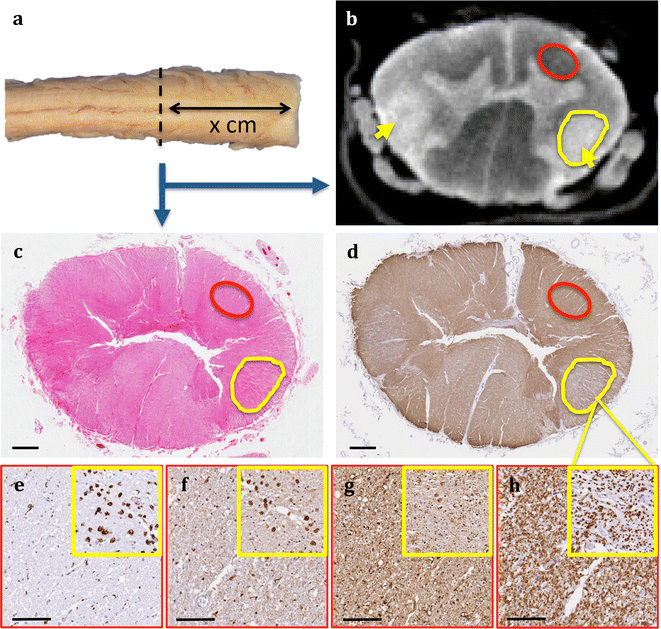
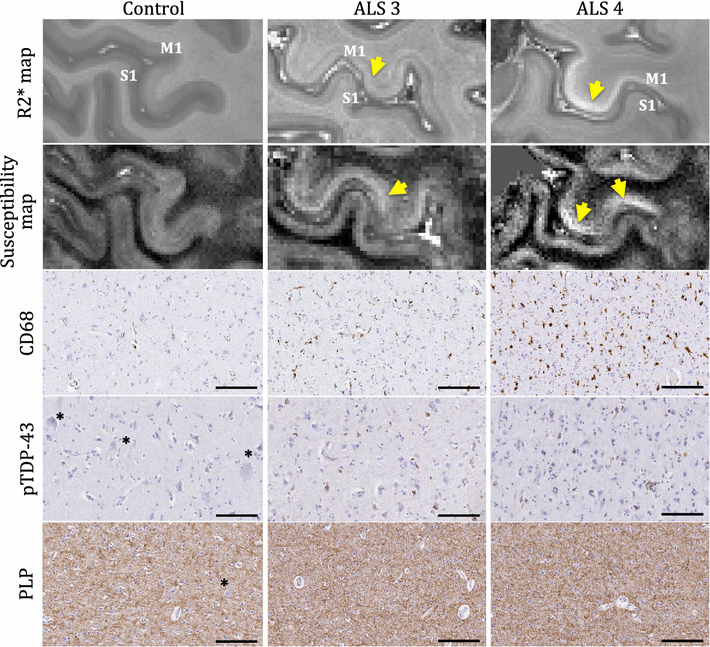
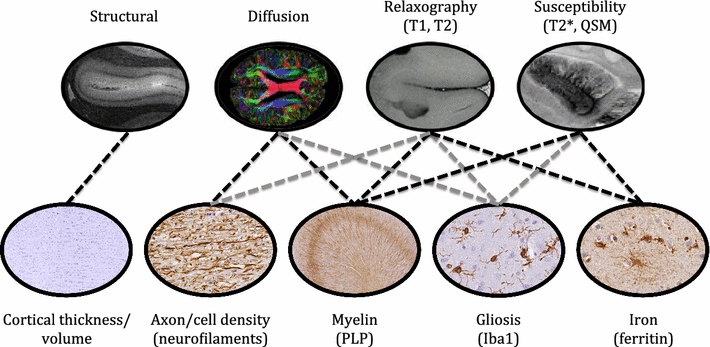
Results: The article delineates a universal whole brain sampling strategy of pathologically relevant grey matter (cortical and subcortical) and white matter tracts of interest suitable for histological evaluation and direct correlation with MRI. A standardised systematic sampling strategy that was compatible with co-registration of images across modalities was established for regions representing phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) patterns that were topographically recognisable with defined neuroanatomical landmarks. Moreover, tractography-guided sampling facilitated accurate delineation of white matter tracts of interest. A digital photography pipeline at various stages of sampling and histological processing was established to account for structural deformations that might impact alignment and registration of histological images to MRI volumes. Combined with quantitative digital histology image analysis, the proposed sampling strategy is suitable for routine implementation in a high-throughput manner for acquisition of large-scale histology datasets. Proof of concept was determined in the spinal cord of an ALS patient where multiple MRI modalities (T1, T2, FA and MD) demonstrated sensitivity to axonal degeneration and associated heightened inflammatory changes in the lateral corticospinal tract. Furthermore, qualitative comparison of R2* and susceptibility maps in the motor cortex of 2 ALS patients demonstrated varying degrees of hyperintense signal changes compared to a control. Upon histological evaluation of the same region, intensity of signal changes in both modalities appeared to correspond primarily to the degree of microglial activation.
Conclusion: The proposed post mortem whole brain sampling methodology enables the accurate intraindividual study of pathological propagation and comparison with quantitative MRI data, to more fully understand the relationship of imaging signal changes with underlying pathophysiology in ALS.
Keywords: Amyotrophic lateral sclerosis; Histology; MRI-histology correlation; Magnetic resonance imaging; Post mortem brain; Systematic sampling.
Figures

Similar articles
-
Structural MRI correlates of amyotrophic lateral sclerosis progression.J Neurol Neurosurg Psychiatry. 2017 Nov;88(11):901-907. doi: 10.1136/jnnp-2016-314337. Epub 2017 May 13. J Neurol Neurosurg Psychiatry. 2017. PMID: 28501822
-
Methods for quantitative susceptibility and R2* mapping in whole post-mortem brains at 7T applied to amyotrophic lateral sclerosis.Neuroimage. 2020 Nov 15;222:117216. doi: 10.1016/j.neuroimage.2020.117216. Epub 2020 Aug 1. Neuroimage. 2020. PMID: 32745677 Free PMC article.
-
Structural explanation of poor prognosis of amyotrophic lateral sclerosis in the non-demented state.Eur J Neurol. 2017 Jan;24(1):122-129. doi: 10.1111/ene.13163. Epub 2016 Oct 18. Eur J Neurol. 2017. PMID: 27753163 Free PMC article.
-
[An autopsy case of amyotrophic lateral screlosis (ALS): magnetic resonance imaging and pathological findings of the pyramidal tract].Rinsho Shinkeigaku. 1997 Sep;37(9):810-6. Rinsho Shinkeigaku. 1997. PMID: 9430996 Review. Japanese.
-
What does imaging reveal about the pathology of amyotrophic lateral sclerosis?Curr Neurol Neurosci Rep. 2015 Jul;15(7):45. doi: 10.1007/s11910-015-0569-6. Curr Neurol Neurosci Rep. 2015. PMID: 26008817 Free PMC article. Review.
Cited by
-
Postmortem 7T MRI for guided histopathology and evaluation of cerebrovascular disease.J Neuropathol Exp Neurol. 2022 Dec 19;82(1):57-70. doi: 10.1093/jnen/nlac103. J Neuropathol Exp Neurol. 2022. PMID: 36343095 Free PMC article.
-
Neuropathology of Pediatric SARS-CoV-2 Infection in the Forensic Setting: Novel Application of Ex Vivo Imaging in Analysis of Brain Microvasculature.Front Neurol. 2022 May 24;13:894565. doi: 10.3389/fneur.2022.894565. eCollection 2022. Front Neurol. 2022. PMID: 35685741 Free PMC article.
-
The Digital Brain Bank, an open access platform for post-mortem imaging datasets.Elife. 2022 Mar 17;11:e73153. doi: 10.7554/eLife.73153. Elife. 2022. PMID: 35297760 Free PMC article.
-
Ex situ perfusion fixation for brain banking: a technical report.Free Neuropathol. 2022 Sep 28;3:22. doi: 10.17879/freeneuropathology-2022-4368. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284166 Free PMC article.
-
Post mortem mapping of connectional anatomy for the validation of diffusion MRI.Neuroimage. 2022 Aug 1;256:119146. doi: 10.1016/j.neuroimage.2022.119146. Epub 2022 Mar 25. Neuroimage. 2022. PMID: 35346838 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- MR/K01014X/1/MRC_/Medical Research Council/United Kingdom
- 202788/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- TURNER/JAN13/944-795/MNDA_/Motor Neurone Disease Association/United Kingdom
- MR/K02213X/1/MRC_/Medical Research Council/United Kingdom
- TURNER/OCT18/989-797/MNDA_/Motor Neurone Disease Association/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

