Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome
- PMID: 29530088
- PMCID: PMC5848565
- DOI: 10.1186/s13071-018-2739-2
Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome
Abstract
Background: Schistosomiasis is a neglected tropical disease burdening millions of people. One drug, praziquantel, is currently used for treatment and control. Clinically relevant drug resistance has not yet been described, but there is considerable heterogeneity in treatment outcomes, ranging from cure to only moderate egg reduction rates. The objectives of this study are to investigate potential worm-induced dysbacteriosis of the gut microbiota and to assess whether a specific microbiome profile could influence praziquantel response.
Methods: Using V3 and V4 regions of 16S rRNA genes, we screened the gut microbiota of 34 Schistosoma mansoni infected and uninfected children from Côte d'Ivoire. From each infected child one pre-treatment, one 24-hour and one 21-day follow-up sample after administering 60 mg/kg praziquantel or placebo, were collected.
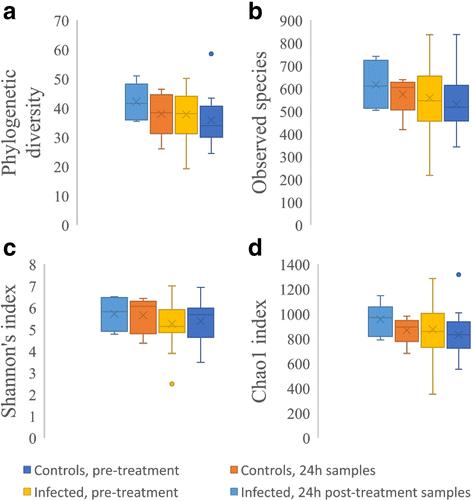
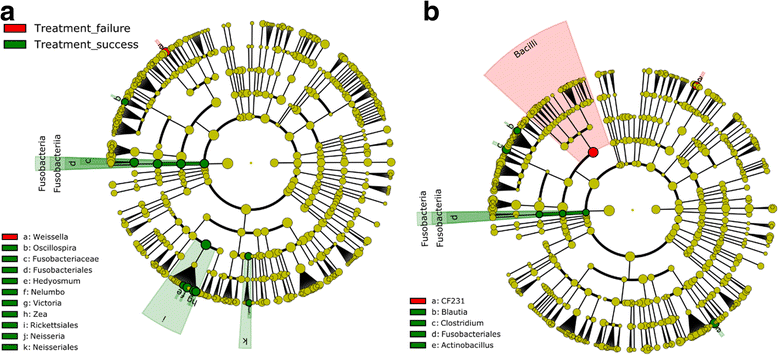
Results: Overall taxonomic profiling and diversity indicators were found to be close to a "healthy" gut structure in all children. Slight overall compositional changes were observed between S. mansoni-infected and non-infected children. Praziquantel treatment was not linked to a major shift in the gut taxonomic profiles, thus reinforcing the good safety profile of the drug by ruling out off-targets effects on the gut microbes.16S rRNA gene of the Fusobacteriales order was significantly more abundant in cured individuals, both at baseline and 24 hours post-treatment. A real-time qPCR confirmed the over-abundance of Fusobacterium spp. in cured children. Fusobacterium spp. abundance could also be correlated with treatment induced S. mansoni egg-reduction.
Conclusions: Our study suggests that neither a S. mansoni infection nor praziquantel administration triggers a significant effect on the microbial composition and that a higher abundance of Fusobacterium spp., before treatment, is associated with higher efficacy of praziquantel in the treatment of S. mansoni infections.
Trial registration: International Standard Randomised Controlled Trial, number ISRCTN15280205 .
Keywords: Gut microbiome; Infectious disease; Microbiome-drug interaction; Microbiome-parasite interaction; Praziquantel; Schistosoma mansoni.
Conflict of interest statement
Ethics approval and consent to participate
Signed assent was obtained from children in addition to a written informed consent from a parent or legal guardian. Participation was voluntary and children had the right to withdraw from the study at any given point in time with no further obligations. Ethical approval for the study was obtained by the National Ethics Committee of the Ministry of Health in Côte d’Ivoire (CNER, reference no. 037/MSLS/CNER-dkn) and the Ethical Committee of Northwestern and Central Switzerland (EKNZ, reference no. 162/2014).
Consent for publication
Consent to publish from the participant (or legal parent or guardian for children) to report individual patient data was obtained.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial.Lancet Glob Health. 2017 Jul;5(7):e688-e698. doi: 10.1016/S2214-109X(17)30187-0. Lancet Glob Health. 2017. PMID: 28619227 Free PMC article. Clinical Trial.
-
Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium.PLoS Negl Trop Dis. 2012;6(12):e1917. doi: 10.1371/journal.pntd.0001917. Epub 2012 Dec 6. PLoS Negl Trop Dis. 2012. PMID: 23236526 Free PMC article.
-
Efficacy and Safety of Moxidectin, Synriam, Synriam-Praziquantel versus Praziquantel against Schistosoma haematobium and S. mansoni Infections: A Randomized, Exploratory Phase 2 Trial.PLoS Negl Trop Dis. 2016 Sep 16;10(9):e0005008. doi: 10.1371/journal.pntd.0005008. eCollection 2016 Sep. PLoS Negl Trop Dis. 2016. PMID: 27636542 Free PMC article. Clinical Trial.
-
Efficacy of single dose praziquantel against Schistosoma mansoni in East Africa: a systematic review and meta-analysis.Sci Rep. 2025 Feb 7;15(1):4642. doi: 10.1038/s41598-024-84621-8. Sci Rep. 2025. PMID: 39920188 Free PMC article.
-
Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children.Parasitology. 2011 Oct;138(12):1593-606. doi: 10.1017/S0031182011001235. Epub 2011 Aug 24. Parasitology. 2011. PMID: 21861945 Free PMC article. Review.
Cited by
-
Effects of Macleaya cordata Extract on Blood Biochemical Indices and Intestinal Flora in Heat-Stressed Mice.Animals (Basel). 2022 Sep 28;12(19):2589. doi: 10.3390/ani12192589. Animals (Basel). 2022. PMID: 36230331 Free PMC article.
-
Does Curcumin Have a Role in the Interaction between Gut Microbiota and Schistosoma mansoni in Mice?Pathogens. 2020 Sep 19;9(9):767. doi: 10.3390/pathogens9090767. Pathogens. 2020. PMID: 32961786 Free PMC article.
-
Alterations in the Gut Microbiota of Tibetan Patients With Echinococcosis.Front Microbiol. 2022 May 9;13:860909. doi: 10.3389/fmicb.2022.860909. eCollection 2022. Front Microbiol. 2022. PMID: 35615499 Free PMC article.
-
Immunomodulatory and biological properties of helminth-derived small molecules: Potential applications in diagnostics and therapeutics.Front Parasitol. 2022 Sep 9;1:984152. doi: 10.3389/fpara.2022.984152. eCollection 2022. Front Parasitol. 2022. PMID: 39816468 Free PMC article. Review.
-
Gut microbiota signatures in Schistosoma japonicum infection-induced liver cirrhosis patients: a case-control study.Infect Dis Poverty. 2021 Mar 26;10(1):43. doi: 10.1186/s40249-021-00821-8. Infect Dis Poverty. 2021. PMID: 33771232 Free PMC article.
References
-
- Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–2191. doi: 10.1016/S0140-6736(15)61340-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

