Exosomal αvβ6 integrin is required for monocyte M2 polarization in prostate cancer
- PMID: 29530483
- PMCID: PMC6081240
- DOI: 10.1016/j.matbio.2018.03.009
Exosomal αvβ6 integrin is required for monocyte M2 polarization in prostate cancer
Abstract
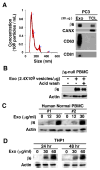
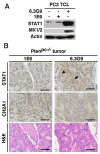
Therapeutic approaches aimed at curing prostate cancer are only partially successful given the occurrence of highly metastatic resistant phenotypes that frequently develop in response to therapies. Recently, we have described αvβ6, a surface receptor of the integrin family as a novel therapeutic target for prostate cancer; this epithelial-specific molecule is an ideal target since, unlike other integrins, it is found in different types of cancer but not in normal tissues. We describe a novel αvβ6-mediated signaling pathway that has profound effects on the microenvironment. We show that αvβ6 is transferred from cancer cells to monocytes, including β6-null monocytes, by exosomes and that monocytes from prostate cancer patients, but not from healthy volunteers, express αvβ6. Cancer cell exosomes, purified via density gradients, promote M2 polarization, whereas αvβ6 down-regulation in exosomes inhibits M2 polarization in recipient monocytes. Also, as evaluated by our proteomic analysis, αvβ6 down-regulation causes a significant increase in donor cancer cells, and their exosomes, of two molecules that have a tumor suppressive role, STAT1 and MX1/2. Finally, using the Ptenpc-/- prostate cancer mouse model, which carries a prostate epithelial-specific Pten deletion, we demonstrate that αvβ6 inhibition in vivo causes up-regulation of STAT1 in cancer cells. Our results provide evidence of a novel mechanism that regulates M2 polarization and prostate cancer progression through transfer of αvβ6 from cancer cells to monocytes through exosomes.
Keywords: Exosomes; M2 polarization; Monocytes; Prostate Cancer; STAT1; αvβ6 integrin.
Copyright © 2017 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Paul H. Weinreb is employee and shareholder of Biogen Idec Inc. The other authors have declared that no conflict of interest exists.
Figures





Similar articles
-
The αvβ6 integrin is transferred intercellularly via exosomes.J Biol Chem. 2015 Feb 20;290(8):4545-4551. doi: 10.1074/jbc.C114.617662. Epub 2015 Jan 7. J Biol Chem. 2015. PMID: 25568317 Free PMC article.
-
Prostate cancer sheds the αvβ3 integrin in vivo through exosomes.Matrix Biol. 2019 Apr;77:41-57. doi: 10.1016/j.matbio.2018.08.004. Epub 2018 Aug 8. Matrix Biol. 2019. PMID: 30098419 Free PMC article.
-
Integrin αvβ6-TGFβ-SOX4 Pathway Drives Immune Evasion in Triple-Negative Breast Cancer.Cancer Cell. 2021 Jan 11;39(1):54-67.e9. doi: 10.1016/j.ccell.2020.12.001. Epub 2020 Dec 31. Cancer Cell. 2021. PMID: 33385331 Free PMC article.
-
The roles of integrin αvβ6 in cancer.Cancer Lett. 2017 Sep 10;403:128-137. doi: 10.1016/j.canlet.2017.06.012. Epub 2017 Jun 17. Cancer Lett. 2017. PMID: 28634043 Review.
-
Role of Exosomes in Prostate Cancer Metastasis.Int J Mol Sci. 2021 Mar 29;22(7):3528. doi: 10.3390/ijms22073528. Int J Mol Sci. 2021. PMID: 33805398 Free PMC article. Review.
Cited by
-
Small extracellular vesicle-mediated ITGB6 siRNA delivery downregulates the αVβ6 integrin and inhibits adhesion and migration of recipient prostate cancer cells.Cancer Biol Ther. 2022 Dec 31;23(1):173-185. doi: 10.1080/15384047.2022.2030622. Cancer Biol Ther. 2022. PMID: 35188070 Free PMC article.
-
Extracellular vesicles as modifiers of antibody-drug conjugate efficacy.J Extracell Vesicles. 2021 Feb;10(4):e12070. doi: 10.1002/jev2.12070. Epub 2021 Feb 13. J Extracell Vesicles. 2021. PMID: 33613875 Free PMC article. Review.
-
Targeting the αVβ3/NgR2 pathway in neuroendocrine prostate cancer.Matrix Biol. 2023 Dec;124:49-62. doi: 10.1016/j.matbio.2023.11.003. Epub 2023 Nov 11. Matrix Biol. 2023. PMID: 37956856 Free PMC article.
-
Exosomal miR-181a-5p derived from SAOS-2 cells promotes macrophages M2 polarization by targeting RORA.Kaohsiung J Med Sci. 2023 Feb;39(2):124-133. doi: 10.1002/kjm2.12623. Epub 2022 Dec 5. Kaohsiung J Med Sci. 2023. PMID: 36468636 Free PMC article.
-
Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies?Front Oncol. 2021 Feb 24;11:620773. doi: 10.3389/fonc.2021.620773. eCollection 2021. Front Oncol. 2021. PMID: 33718177 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. - PubMed
-
- Gerlinger M, Catto JW, Orntoft TF, Real FX, Zwarthoff EC, Swanton C. Intratumour Heterogeneity in Urologic Cancers: From Molecular Evidence to Clinical Implications. Eur Urol. 2014;67(4):729–737. - PubMed
-
- Alva A, Hussain M. The changing natural history of metastatic prostate cancer. Cancer J. 2013;19(1):19–24. - PubMed
-
- Culig Z. Targeting the androgen receptor in prostate cancer. Expert Opin Pharmacother. 2014;15(10):1427–1437. - PubMed
-
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20(3):203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

