Tapering Courses of Oral Vancomycin Induce Persistent Disruption of the Microbiota That Provide Colonization Resistance to Clostridium difficile and Vancomycin-Resistant Enterococci in Mice
- PMID: 29530853
- PMCID: PMC5923165
- DOI: 10.1128/AAC.02237-17
Tapering Courses of Oral Vancomycin Induce Persistent Disruption of the Microbiota That Provide Colonization Resistance to Clostridium difficile and Vancomycin-Resistant Enterococci in Mice
Abstract
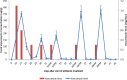
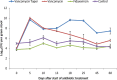
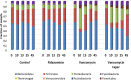
Vancomycin taper regimens are commonly used for the treatment of recurrent Clostridium difficile infections. One rationale for tapering and pulsing of the dose at the end of therapy is to reduce the selective pressure of vancomycin on the indigenous intestinal microbiota. Here, we used a mouse model to test the hypothesis that the indigenous microbiota that provide colonization resistance against C. difficile and vancomycin-resistant enterococci (VRE) is repopulated during tapering courses of vancomycin. Mice were treated orally with vancomycin daily for 10 days, vancomycin in a tapering dose for 42 days, fidaxomicin for 10 days, or saline. To assess colonization resistance, subsets of mice were challenged with 104 CFU of C. difficile or VRE at multiple time points during and after completion of treatment. The impact of the treatments on the microbiome was measured by cultures, real-time PCR for selected anaerobic bacteria, and deep sequencing. Vancomycin taper-treated mice developed alterations of the microbiota and disruption of colonization resistance that was persistent 18 days after treatment. In contrast, mice treated with a 10-day course of vancomycin exhibited recovery of the microbiota and of colonization resistance by 15 days after treatment, and fidaxomicin-treated mice maintained intact colonization resistance. These findings demonstrate that alteration of the indigenous microbiota responsible for colonization resistance to C. difficile and VRE persist during and after completion of tapering courses of vancomycin.
Keywords: colonization resistance; oral vancomycin.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Effect of Fidaxomicin versus Vancomycin on Susceptibility to Intestinal Colonization with Vancomycin-Resistant Enterococci and Klebsiella pneumoniae in Mice.Antimicrob Agents Chemother. 2016 Jun 20;60(7):3988-93. doi: 10.1128/AAC.02590-15. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27090175 Free PMC article.
-
Impact of Oral Fidaxomicin Administration on the Intestinal Microbiota and Susceptibility to Clostridium difficile Colonization in Mice.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02112-17. doi: 10.1128/AAC.02112-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463537 Free PMC article.
-
Cadazolid Does Not Promote Intestinal Colonization of Vancomycin-Resistant Enterococci in Mice.Antimicrob Agents Chemother. 2015 Oct 26;60(1):628-31. doi: 10.1128/AAC.01923-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26503650 Free PMC article.
-
Fidaxomicin: a novel macrocyclic antibiotic for the treatment of Clostridium difficile infection.Am J Health Syst Pharm. 2012 Jun 1;69(11):933-43. doi: 10.2146/ajhp110371. Am J Health Syst Pharm. 2012. PMID: 22610025 Review.
-
Fidaxomicin (OPT-80) for the treatment of Clostridium difficile infection.Expert Opin Pharmacother. 2010 Jun;11(9):1569-78. doi: 10.1517/14656566.2010.485614. Expert Opin Pharmacother. 2010. PMID: 20446864 Review.
Cited by
-
Biofilm Formation of Clostridioides difficile, Toxin Production and Alternatives to Conventional Antibiotics in the Treatment of CDI.Microorganisms. 2023 Aug 26;11(9):2161. doi: 10.3390/microorganisms11092161. Microorganisms. 2023. PMID: 37764005 Free PMC article. Review.
-
Efficacy of prolonged tapered and pulsed vancomycin regimen on recurrent Clostridioides difficile infection in the Japanese setting: a case control study.J Pharm Health Care Sci. 2019 Aug 8;5:19. doi: 10.1186/s40780-019-0147-1. eCollection 2019. J Pharm Health Care Sci. 2019. PMID: 31410293 Free PMC article.
-
Impact of tebipenem pivoxil on the intestinal microbiota and on establishment of colonization with carbapenem-resistant Klebsiella pneumoniae in mice.Microbiol Spectr. 2025 Mar 14;13(5):e0234624. doi: 10.1128/spectrum.02346-24. Online ahead of print. Microbiol Spectr. 2025. PMID: 40084911 Free PMC article.
-
Pulsed dosing and extended daily dosing of oral vancomycin do not facilitate clearance of Clostridioides difficile colonization in mice.Antimicrob Agents Chemother. 2024 Jan 10;68(1):e0090323. doi: 10.1128/aac.00903-23. Epub 2023 Dec 14. Antimicrob Agents Chemother. 2024. PMID: 38095427 Free PMC article.
-
Why Does Doxycycline Pose a Relatively Low Risk for Promotion of Clostridioides difficile Infection?Pathog Immun. 2022 Jun 21;7(1):81-94. doi: 10.20411/pai.v7i1.512. eCollection 2022. Pathog Immun. 2022. PMID: 35800258 Free PMC article.
References
-
- Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, Krulicki W. 2012. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis 55(Suppl 2):S132–S142. doi: 10.1093/cid/cis338. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

