Endosomal-Lysosomal Cholesterol Sequestration by U18666A Differentially Regulates Amyloid Precursor Protein (APP) Metabolism in Normal and APP-Overexpressing Cells
- PMID: 29530923
- PMCID: PMC5954187
- DOI: 10.1128/MCB.00529-17
Endosomal-Lysosomal Cholesterol Sequestration by U18666A Differentially Regulates Amyloid Precursor Protein (APP) Metabolism in Normal and APP-Overexpressing Cells
Abstract
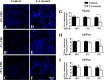
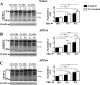
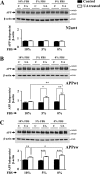
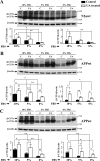
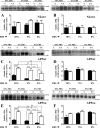
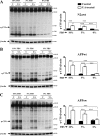
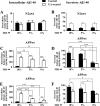
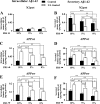
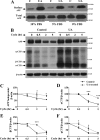
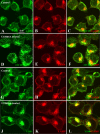
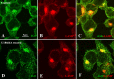
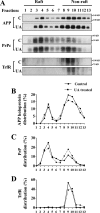
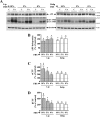
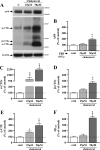
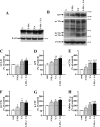
Amyloid β (Aβ) peptide, derived from amyloid precursor protein (APP), plays a critical role in the development of Alzheimer's disease. Current evidence indicates that altered levels or subcellular distribution of cholesterol can regulate Aβ production and clearance, but it remains unclear how cholesterol sequestration within the endosomal-lysosomal (EL) system can influence APP metabolism. Thus, we evaluated the effects of U18666A, which triggers cholesterol redistribution within the EL system, on mouse N2a cells expressing different levels of APP in the presence or absence of extracellular cholesterol and lipids provided by fetal bovine serum (FBS). Our results reveal that U18666A and FBS differentially increase the levels of APP and its cleaved products, the α-, β-, and η-C-terminal fragments, in N2a cells expressing normal levels of mouse APP (N2awt), higher levels of human wild-type APP (APPwt), or "Swedish" mutant APP (APPsw). The cellular levels of Aβ1-40/Aβ1-42 were markedly increased in U18666A-treated APPwt and APPsw cells. Our studies further demonstrate that APP and its cleaved products are partly accumulated in the lysosomes, possibly due to decreased clearance. Finally, we show that autophagy inhibition plays a role in mediating U18666A effects. Collectively, these results suggest that altered levels and distribution of cholesterol and lipids can differentially regulate APP metabolism depending on the nature of APP expression.
Keywords: Alzheimer's disease; amyloid precursor protein; delipidation; endosomal-lysosomal system; lipid raft; β-amyloid; β-secretase; γ-secretase.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Effects of cholesterol transport inhibitor U18666A on APP metabolism in rat primary astrocytes.Glia. 2017 Nov;65(11):1728-1743. doi: 10.1002/glia.23191. Epub 2017 Jul 19. Glia. 2017. PMID: 28722194
-
APP overexpression in the absence of NPC1 exacerbates metabolism of amyloidogenic proteins of Alzheimer's disease.Hum Mol Genet. 2015 Dec 15;24(24):7132-50. doi: 10.1093/hmg/ddv413. Epub 2015 Oct 3. Hum Mol Genet. 2015. PMID: 26433932 Free PMC article.
-
The Effects of Extracellular Serum Concentration on APP Processing in Npc1-Deficient APP-Overexpressing N2a Cells.Mol Neurobiol. 2018 Jul;55(7):5757-5766. doi: 10.1007/s12035-017-0799-5. Epub 2017 Oct 19. Mol Neurobiol. 2018. PMID: 29052144
-
Dysregulation of intracellular trafficking and endosomal sorting in Alzheimer's disease: controversies and unanswered questions.Biochem J. 2016 Jul 15;473(14):1977-93. doi: 10.1042/BCJ20160147. Biochem J. 2016. PMID: 27407168 Review.
-
Role of amyloid beta in lipid homeostasis.Biochim Biophys Acta. 2010 Aug;1801(8):966-74. doi: 10.1016/j.bbalip.2010.05.002. Epub 2010 May 7. Biochim Biophys Acta. 2010. PMID: 20452461 Review.
Cited by
-
Role of cholesterol and sphingolipids in brain development and neurological diseases.Lipids Health Dis. 2019 Jan 25;18(1):26. doi: 10.1186/s12944-019-0965-z. Lipids Health Dis. 2019. PMID: 30683111 Free PMC article. Review.
-
Cholesterol-dependent amyloid β production: space for multifarious interactions between amyloid precursor protein, secretases, and cholesterol.Cell Biosci. 2023 Sep 13;13(1):171. doi: 10.1186/s13578-023-01127-y. Cell Biosci. 2023. PMID: 37705117 Free PMC article. Review.
-
Organelle-targeted Laurdans measure heterogeneity in subcellular membranes and their responses to saturated lipid stress.bioRxiv [Preprint]. 2024 Apr 20:2024.04.16.589828. doi: 10.1101/2024.04.16.589828. bioRxiv. 2024. Update in: ACS Chem Biol. 2024 Aug 16;19(8):1773-1785. doi: 10.1021/acschembio.4c00249. PMID: 38659784 Free PMC article. Updated. Preprint.
-
Amyloid-beta metabolism in age-related neurocardiovascular diseases.Eur Heart J. 2025 Jan 16;46(3):250-272. doi: 10.1093/eurheartj/ehae655. Eur Heart J. 2025. PMID: 39527015 Free PMC article. Review.
-
Cholesterol as a key player in amyloid β-mediated toxicity in Alzheimer's disease.Front Mol Neurosci. 2022 Aug 25;15:937056. doi: 10.3389/fnmol.2022.937056. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36090253 Free PMC article. Review.
References
-
- Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. 2013. Impaired autophagy and APP processing in Alzheimer's disease: the potential role of Beclin 1 interactome. Prog Neurobiol 106-107:33–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
