Regulation of RUNX1 dosage is crucial for efficient blood formation from hemogenic endothelium
- PMID: 29530939
- PMCID: PMC5868988
- DOI: 10.1242/dev.149419
Regulation of RUNX1 dosage is crucial for efficient blood formation from hemogenic endothelium
Abstract
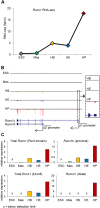
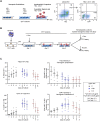
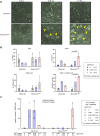
During ontogeny, hematopoietic stem and progenitor cells arise from hemogenic endothelium through an endothelial-to-hematopoietic transition that is strictly dependent on the transcription factor RUNX1. Although it is well established that RUNX1 is essential for the onset of hematopoiesis, little is known about the role of RUNX1 dosage specifically in hemogenic endothelium and during the endothelial-to-hematopoietic transition. Here, we used the mouse embryonic stem cell differentiation system to determine if and how RUNX1 dosage affects hemogenic endothelium differentiation. The use of inducible Runx1 expression combined with alterations in the expression of the RUNX1 co-factor CBFβ allowed us to evaluate a wide range of RUNX1 levels. We demonstrate that low RUNX1 levels are sufficient and necessary to initiate an effective endothelial-to-hematopoietic transition. Subsequently, RUNX1 is also required to complete the endothelial-to-hematopoietic transition and to generate functional hematopoietic precursors. In contrast, elevated levels of RUNX1 are able to drive an accelerated endothelial-to-hematopoietic transition, but the resulting cells are unable to generate mature hematopoietic cells. Together, our results suggest that RUNX1 dosage plays a pivotal role in hemogenic endothelium maturation and the establishment of the hematopoietic system.
Keywords: CBFβ; Dosage; EHT; Hemogenic endothelium; RUNX1; SOX7.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



Similar articles
-
Developmental trajectory of prehematopoietic stem cell formation from endothelium.Blood. 2020 Aug 13;136(7):845-856. doi: 10.1182/blood.2020004801. Blood. 2020. PMID: 32392346 Free PMC article.
-
Interplay between SOX7 and RUNX1 regulates hemogenic endothelial fate in the yolk sac.Development. 2016 Dec 1;143(23):4341-4351. doi: 10.1242/dev.140970. Epub 2016 Oct 17. Development. 2016. PMID: 27802172
-
Efficient hemogenic endothelial cell specification by RUNX1 is dependent on baseline chromatin accessibility of RUNX1-regulated TGFβ target genes.Genes Dev. 2021 Nov 1;35(21-22):1475-1489. doi: 10.1101/gad.348738.121. Epub 2021 Oct 21. Genes Dev. 2021. PMID: 34675061 Free PMC article.
-
Regulation of Hemogenic Endothelial Cell Development and Function.Annu Rev Physiol. 2021 Feb 10;83:17-37. doi: 10.1146/annurev-physiol-021119-034352. Epub 2020 Oct 9. Annu Rev Physiol. 2021. PMID: 33035429 Free PMC article. Review.
-
The Role of Runx1 in Embryonic Blood Cell Formation.Adv Exp Med Biol. 2017;962:47-64. doi: 10.1007/978-981-10-3233-2_4. Adv Exp Med Biol. 2017. PMID: 28299650 Free PMC article. Review.
Cited by
-
Developmental trajectory of prehematopoietic stem cell formation from endothelium.Blood. 2020 Aug 13;136(7):845-856. doi: 10.1182/blood.2020004801. Blood. 2020. PMID: 32392346 Free PMC article.
-
RUNX1 Dosage in Development and Cancer.Mol Cells. 2020 Feb 29;43(2):126-138. doi: 10.14348/molcells.2019.0301. Mol Cells. 2020. PMID: 31991535 Free PMC article. Review.
-
Blood making: learning what to put into the dish.F1000Res. 2020 Jan 23;9:F1000 Faculty Rev-38. doi: 10.12688/f1000research.21245.1. eCollection 2020. F1000Res. 2020. PMID: 32047610 Free PMC article. Review.
-
Spatiotemporal and Functional Heterogeneity of Hematopoietic Stem Cell-Competent Hemogenic Endothelial Cells in Mouse Embryos.Front Cell Dev Biol. 2021 Aug 11;9:699263. doi: 10.3389/fcell.2021.699263. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458261 Free PMC article.
-
RUNX1 isoforms regulate RUNX1 and target genes differentially in platelets-megakaryocytes: association with clinical cardiovascular events.J Thromb Haemost. 2024 Dec;22(12):3581-3598. doi: 10.1016/j.jtha.2024.07.032. Epub 2024 Aug 23. J Thromb Haemost. 2024. PMID: 39181539
References
-
- Banno K., Omori S., Hirata K., Nawa N., Nakagawa N., Nishimura K., Ohtaka M., Nakanishi M., Sakuma T., Yamamoto T. et al. (2016). Systematic cellular disease models reveal synergistic interaction of trisomy 21 and GATA1 mutations in hematopoietic abnormalities. Cell Rep. 15, 1228-1241. 10.1016/j.celrep.2016.04.031 - DOI - PubMed
-
- Bee T., Swiers G., Muroi S., Pozner A., Nottingham W., Santos A. C., Li P. S., Taniuchi I. and de Bruijn M. F. T. R. (2010). Nonredundant roles for Runx1 alternative promoters reflect their activity at discrete stages of developmental hematopoiesis. Blood 115, 3042-3050. 10.1182/blood-2009-08-238626 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

