Regulation of hepatic gluconeogenesis by nuclear factor Y transcription factor in mice
- PMID: 29530977
- PMCID: PMC5961052
- DOI: 10.1074/jbc.RA117.000508
Regulation of hepatic gluconeogenesis by nuclear factor Y transcription factor in mice
Abstract
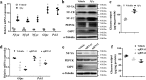
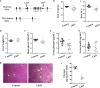
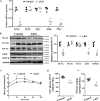
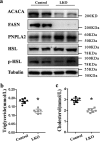
Hepatic gluconeogenesis is essential to maintain blood glucose levels, and its abnormal activation leads to hyperglycemia and type 2 diabetes. However, the molecular mechanisms in the regulation of hepatic gluconeogenesis remain to be fully defined. In this study, using murine hepatocytes and a liver-specific knockout mouse model, we explored the physiological role of nuclear factor Y (NF-Y) in regulating hepatic glucose metabolism and the underlying mechanism. We found that NF-Y targets the gluconeogenesis pathway in the liver. Hepatic NF-Y expression was effectively induced by cAMP, glucagon, and fasting in vivo Lentivirus-mediated NF-Y overexpression in Hepa1-6 hepatocytes markedly raised the gluconeogenic gene expression and cellular glucose production compared with empty vector control cells. Conversely, CRISPR/Cas9-mediated knockdown of NF-Y subunit A (NF-YA) attenuated gluconeogenic gene expression and glucose production. We also provide evidence indicating that CRE-loxP-mediated, liver-specific NF-YA knockout compromises hepatic glucose production. Mechanistically, luciferase reporter gene assays and ChIP analysis indicated that NF-Y activates transcription of the gluconeogenic genes Pck1 and G6pc, by encoding phosphoenolpyruvate carboxykinase (PEPCK) and the glucose-6-phosphatase catalytic subunit (G6Pase), respectively, via directly binding to the CCAAT regulatory sequence motif in their promoters. Of note, NF-Y enhanced gluconeogenesis by interacting with cAMP-responsive element-binding protein (CREB). Overall, our results reveal a previously unrecognized physiological function of NF-Y in controlling glucose metabolism by up-regulating the gluconeogenic genes Pck1 and G6pc Modulation of hepatic NF-Y expression may therefore offer an attractive therapeutic approach to manage type 2 diabetes.
Keywords: CREB; G6Pase; PEPCK; diabetes; gene knockout; glucagon; gluconeogenesis; mouse; nuclear factor Y; transcription regulation.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Comment in
-
Managing the sugar factory: A new feather in the cap for nuclear factor Y.J Biol Chem. 2018 May 18;293(20):7905-7906. doi: 10.1074/jbc.H118.002885. J Biol Chem. 2018. PMID: 29777018 Free PMC article.
Similar articles
-
Zbtb7c is a critical gluconeogenic transcription factor that induces glucose-6-phosphatase and phosphoenylpyruvate carboxykinase 1 genes expression during mice fasting.Biochim Biophys Acta Gene Regul Mech. 2019 Jun;1862(6):643-656. doi: 10.1016/j.bbagrm.2019.04.001. Epub 2019 Apr 5. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 30959128
-
Regulation of Hepatic Gluconeogenesis by Nuclear Receptor Coactivator 6.Mol Cells. 2022 Apr 30;45(4):180-192. doi: 10.14348/molcells.2022.2222. Mol Cells. 2022. PMID: 35258009 Free PMC article.
-
CREB-upregulated lncRNA MEG3 promotes hepatic gluconeogenesis by regulating miR-302a-3p-CRTC2 axis.J Cell Biochem. 2019 Mar;120(3):4192-4202. doi: 10.1002/jcb.27706. Epub 2018 Sep 27. J Cell Biochem. 2019. PMID: 30260029
-
Hormonal regulation of gluconeogenic gene transcription in the liver.J Biosci. 2010 Sep;35(3):473-84. doi: 10.1007/s12038-010-0052-0. J Biosci. 2010. PMID: 20826956 Review.
-
CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis.BMB Rep. 2013 Dec;46(12):567-74. doi: 10.5483/bmbrep.2013.46.12.248. BMB Rep. 2013. PMID: 24238363 Free PMC article. Review.
Cited by
-
Pancreatic β-cell failure, clinical implications, and therapeutic strategies in type 2 diabetes.Chin Med J (Engl). 2024 Apr 5;137(7):791-805. doi: 10.1097/CM9.0000000000003034. Epub 2024 Mar 13. Chin Med J (Engl). 2024. PMID: 38479993 Free PMC article. Review.
-
H2S-Synthesizing Enzymes Are Putative Determinants in Lung Cancer Management toward Personalized Medicine.Antioxidants (Basel). 2023 Dec 28;13(1):51. doi: 10.3390/antiox13010051. Antioxidants (Basel). 2023. PMID: 38247476 Free PMC article.
-
Aging-induced short-chain acyl-CoA dehydrogenase promotes age-related hepatic steatosis by suppressing lipophagy.Aging Cell. 2024 Oct;23(10):e14256. doi: 10.1111/acel.14256. Epub 2024 Jun 19. Aging Cell. 2024. PMID: 38898632 Free PMC article.
-
Effects of Genetic Variants of Nuclear Receptor Y on the Risk of Type 2 Diabetes Mellitus.J Diabetes Res. 2019 May 7;2019:4902301. doi: 10.1155/2019/4902301. eCollection 2019. J Diabetes Res. 2019. PMID: 31205951 Free PMC article.
-
NF-Y Overexpression in Liver Hepatocellular Carcinoma (HCC).Int J Mol Sci. 2020 Dec 1;21(23):9157. doi: 10.3390/ijms21239157. Int J Mol Sci. 2020. PMID: 33271832 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

