The apical anion exchanger Slc26a6 promotes oxalate secretion by murine submandibular gland acinar cells
- PMID: 29530983
- PMCID: PMC5925796
- DOI: 10.1074/jbc.RA118.002378
The apical anion exchanger Slc26a6 promotes oxalate secretion by murine submandibular gland acinar cells
Abstract
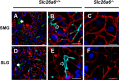
The solute carrier family 26 (SLC26) gene family encodes at least 10 different anion exchangers. SLC26 member 6 (SLC26A6 or CFEX/PAT-1) and the cystic fibrosis transmembrane conductance regulator (CFTR) co-localize to the apical membrane of pancreatic duct cells, where they act in concert to drive HCO3- and fluid secretion. In contrast, in the small intestine, SLC26A6 serves as the major pathway for oxalate secretion. However, little is known about the function of Slc26a6 in murine salivary glands. Here, RNA sequencing-based transcriptional profiling and Western blots revealed that Slc26a6 is highly expressed in mouse submandibular and sublingual salivary glands. Slc26a6 localized to the apical membrane of salivary gland acinar cells with no detectable immunostaining in the ducts. CHO-K1 cells transfected with mouse Slc26a6 exchanged Cl- for oxalate and HCO3-, whereas two other anion exchangers known to be expressed in salivary gland acinar cells, Slc4a4 and Slc4a9, mediated little, if any, Cl-/oxalate exchange. Of note, both Cl-/oxalate exchange and Cl-/HCO3- exchange were significantly reduced in acinar cells isolated from the submandibular glands of Slc26a6-/- mice. Oxalate secretion in submandibular saliva also decreased significantly in Slc26a6-/- mice, but HCO3- secretion was unaffected. Taken together, our findings indicate that Slc26a6 is located at the apical membrane of salivary gland acinar cells, where it mediates Cl-/oxalate exchange and plays a critical role in the secretion of oxalate into saliva.
Keywords: anion transport; epithelial cell; exchanger; glycoprotein; physiology.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

