Substrate recognition and mechanism revealed by ligand-bound polyphosphate kinase 2 structures
- PMID: 29531036
- PMCID: PMC5879653
- DOI: 10.1073/pnas.1710741115
Substrate recognition and mechanism revealed by ligand-bound polyphosphate kinase 2 structures
Abstract
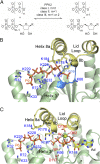
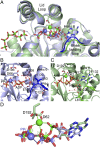
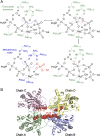
Inorganic polyphosphate is a ubiquitous, linear biopolymer built of up to thousands of phosphate residues that are linked by energy-rich phosphoanhydride bonds. Polyphosphate kinases of the family 2 (PPK2) use polyphosphate to catalyze the reversible phosphorylation of nucleotide phosphates and are highly relevant as targets for new pharmaceutical compounds and as biocatalysts for cofactor regeneration. PPK2s can be classified based on their preference for nucleoside mono- or diphosphates or both. The detailed mechanism of PPK2s and the molecular basis for their substrate preference is unclear, which is mainly due to the lack of high-resolution structures with substrates or substrate analogs. Here, we report the structural analysis and comparison of a class I PPK2 (ADP-phosphorylating) and a class III PPK2 (AMP- and ADP-phosphorylating), both complexed with polyphosphate and/or nucleotide substrates. Together with complementary biochemical analyses, these define the molecular basis of nucleotide specificity and are consistent with a Mg2+ catalyzed in-line phosphoryl transfer mechanism. This mechanistic insight will guide the development of PPK2 inhibitors as potential antibacterials or genetically modified PPK2s that phosphorylate alternative substrates.
Keywords: enzyme structure; kinase; kinetics; polyphosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Class III Polyphosphate Kinase 2 Enzymes Catalyze the Pyrophosphorylation of Adenosine-5'-Monophosphate.Chembiochem. 2019 Dec 2;20(23):2961-2967. doi: 10.1002/cbic.201900303. Epub 2019 Aug 22. Chembiochem. 2019. PMID: 31206993
-
Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria.Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):17730-5. doi: 10.1073/pnas.0807563105. Epub 2008 Nov 10. Proc Natl Acad Sci U S A. 2008. PMID: 19001261 Free PMC article.
-
Several Polyphosphate Kinase 2 Enzymes Catalyse the Production of Adenosine 5'-Polyphosphates.Chembiochem. 2019 Apr 15;20(8):1019-1022. doi: 10.1002/cbic.201800704. Epub 2019 Feb 28. Chembiochem. 2019. PMID: 30549179
-
Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence.Int J Mol Sci. 2022 Jan 8;23(2):670. doi: 10.3390/ijms23020670. Int J Mol Sci. 2022. PMID: 35054854 Free PMC article. Review.
-
Enzymes of inorganic polyphosphate metabolism.Prog Mol Subcell Biol. 2013;54:39-63. doi: 10.1007/978-3-642-41004-8_3. Prog Mol Subcell Biol. 2013. PMID: 24420710 Review.
Cited by
-
Structural Insights into Broad-Range Polyphosphate Kinase 2-II Enzymes Applicable for Pyrimidine Nucleoside Diphosphate Synthesis.Chembiochem. 2025 Feb 3;26(5):e202400970. doi: 10.1002/cbic.202400970. Epub 2025 Feb 4. Chembiochem. 2025. PMID: 39846220 Free PMC article.
-
Selectivity in Enzymatic Phosphorus Recycling from Biopolymers: Isotope Effect, Reactivity Kinetics, and Molecular Docking with Fungal and Plant Phosphatases.Environ Sci Technol. 2022 Nov 15;56(22):16441-16452. doi: 10.1021/acs.est.2c04948. Epub 2022 Oct 25. Environ Sci Technol. 2022. PMID: 36283689 Free PMC article.
-
A Dual-Specificity Inhibitor Targets Polyphosphate Kinase 1 and 2 Enzymes To Attenuate Virulence of Pseudomonas aeruginosa.mBio. 2021 Jun 29;12(3):e0059221. doi: 10.1128/mBio.00592-21. Epub 2021 Jun 15. mBio. 2021. PMID: 34126765 Free PMC article.
-
Multi-enzyme catalysed processes using purified and whole-cell biocatalysts towards a 1,3,4-substituted tetrahydroisoquinoline.RSC Adv. 2023 Mar 29;13(15):10097-10109. doi: 10.1039/d3ra01210g. eCollection 2023 Mar 27. RSC Adv. 2023. PMID: 37006360 Free PMC article.
-
Molecular characterization of CHAD domains as inorganic polyphosphate-binding modules.Life Sci Alliance. 2019 May 27;2(3):e201900385. doi: 10.26508/lsa.201900385. Print 2019 Jun. Life Sci Alliance. 2019. PMID: 31133615 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

