Lateral cerebellum is preferentially sensitive to high sonic hedgehog signaling and medulloblastoma formation
- PMID: 29531057
- PMCID: PMC5879676
- DOI: 10.1073/pnas.1717815115
Lateral cerebellum is preferentially sensitive to high sonic hedgehog signaling and medulloblastoma formation
Abstract
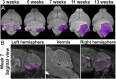
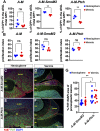
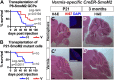
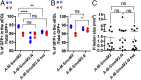
The main cell of origin of the Sonic hedgehog (SHH) subgroup of medulloblastoma (MB) is granule cell precursors (GCPs), a SHH-dependent transient amplifying population in the developing cerebellum. SHH-MBs can be further subdivided based on molecular and clinical parameters, as well as location because SHH-MBs occur preferentially in the lateral cerebellum (hemispheres). Our analysis of adult patient data suggests that tumors with Smoothened (SMO) mutations form more specifically in the hemispheres than those with Patched 1 (PTCH1) mutations. Using sporadic mouse models of SHH-MB with the two mutations commonly seen in adult MB, constitutive activation of Smo (SmoM2) or loss-of-Ptch1, we found that regardless of timing of induction or type of mutation, tumors developed primarily in the hemispheres, with SmoM2-mutants indeed showing a stronger specificity. We further uncovered that GCPs in the hemispheres are more susceptible to high-level SHH signaling compared with GCPs in the medial cerebellum (vermis), as more SmoM2 or Ptch1-mutant hemisphere cells remain undifferentiated and show increased tumorigenicity when transplanted. Finally, we identified location-specific GCP gene-expression profiles, and found that deletion of the genes most highly expressed in the hemispheres (Nr2f2) or vermis (Engrailed1) showed opposing effects on GCP differentiation. Our studies thus provide insights into intrinsic differences within GCPs that impact on SHH-MB progression.
Keywords: En1; MRI; Nr2f2; cerebellar hemispheres; granule cell precursors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8:340–351. - PubMed
-
- Louis DN, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

