Gpr97 Exacerbates AKI by Mediating Sema3A Signaling
- PMID: 29531097
- PMCID: PMC5967783
- DOI: 10.1681/ASN.2017080932
Gpr97 Exacerbates AKI by Mediating Sema3A Signaling
Abstract
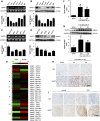
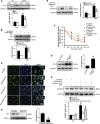
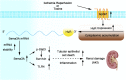
Background G protein-coupled receptors (GPCRs) participate in a variety of physiologic functions, and several GPCRs have critical physiologic and pathophysiologic roles in the regulation of renal function. We investigated the role of Gpr97, a newly identified member of the adhesion GPCR family, in AKI.Methods AKI was induced by ischemia-reperfusion or cisplatin treatment in Gpr97-deficient mice. We assessed renal injury in these models and in patients with acute tubular necrosis by histologic examination, and we conducted microarray analysis and in vitro assays to determine the molecular mechanisms of Gpr97 function.Results Gpr97 was upregulated in the kidneys from mice with AKI and patients with biopsy-proven acute tubular necrosis compared with healthy controls. In AKI models, Gpr97-deficient mice had significantly less renal injury and inflammation than wild-type mice. Gpr97 deficiency also attenuated the AKI-induced expression of semaphorin 3A (Sema3A), a potential early diagnostic biomarker of renal injury. In NRK-52E cells subjected to oxygen-glucose deprivation, siRNA-mediated knockdown of Gpr97 further increased the expression of survivin and phosphorylated STAT3 and reduced toll-like receptor 4 expression. Cotreatment with recombinant murine Sema3A protein counteracted these effects. Finally, additional in vivo and in vitro studies, including electrophoretic mobility shift assays and luciferase reporter assays, showed that Gpr97 deficiency attenuates ischemia-reperfusion-induced expression of the RNA-binding protein human antigen R, which post-transcriptionally regulates Sema3A expression.Conclusions Gpr97 is an important mediator of AKI, and pharmacologic targeting of Gpr97-mediated Sema3A signaling at multiple levels may provide a novel approach for the treatment of AKI.
Keywords: acute renal failure; cisplatin nephrotoxicity; ischemia-reperfusion; renal cell biology; renal tubular epithelial cells; tubular epithelium.
Copyright © 2018 by the American Society of Nephrology.
Figures






References
-
- Jang HR, Rabb H: Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015 - PubMed
-
- Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 - PubMed
-
- Kishore A, Hall RA: Versatile signaling activity of adhesion GPCRs. Handb Exp Pharmacol 234: 127–146, 2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

