Use of Asthma APGAR Tools in Primary Care Practices: A Cluster-Randomized Controlled Trial
- PMID: 29531100
- PMCID: PMC5847347
- DOI: 10.1370/afm.2179
Use of Asthma APGAR Tools in Primary Care Practices: A Cluster-Randomized Controlled Trial
Abstract
Purpose: The purpose of this study was to assess patient and practice outcomes after introducing the Asthma APGAR (Activities, Persistent, triGGers, Asthma medications, Response to therapy) tools into primary care practices.
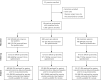
Methods: We used a pragmatic cluster-randomized controlled design in 18 US family medicine and pediatric practices to compare outcomes in patients with persistent asthma aged 5 to 45 years after introduction of the Asthma APGAR tools vs usual care. Patient outcomes included asthma control, quality of life, and emergency department (ED), urgent care, and inpatient hospital visits. The practice outcome was adherence to asthma guidelines.
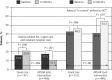
Results: We enrolled 1,066 patients: 245 children, 174 adolescents, and 647 adults. Sixty-five percent (692 patients) completed both baseline and 12-month questionnaires, allowing analysis for patient-reported outcomes. Electronic health record data were available for 1,063 patients (99.7%) for practice outcomes. The proportion of patients reporting an asthma-related ED, urgent care, or hospital visit in the final 6 months of the study was lower in the APGAR practices vs usual care practices (10.6% vs 20.9%, P = .004). The percentage of patients with "in control" asthma increased more between baseline and 1 year in the APGAR group vs usual care group (13.5% vs 3.4%, P =.0001 vs P =.86) with a trend toward better control scores and asthma-related quality of life in the former at 1 year (P ≤.06 and P = .06, respectively). APGAR practices improved their adherence to 3 or more guideline elements compared with usual care practices (20.7% increase vs 1.9% decrease, P = .001).
Conclusions: Introduction of the Asthma APGAR tools improves rates of asthma control; reduces asthma-related ED, urgent care, and hospital visits; and increases practices' adherence to asthma management guidelines.
Trial registration: ClinicalTrials.gov NCT01446315.
Keywords: asthma; asthma control; asthma management; asthma tool; guideline; implementation; outcomes; practice-based research; pragmatic research; primary care; protocol; randomized clinical trial.
© 2018 Annals of Family Medicine, Inc.
Conflict of interest statement
Conflicts of interest: Barbara P. Yawn served on asthma and COPD advisory boards for Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Teva. Young Juhn received support for asthma research from Genentech. All other authors report no conflicts of interest.
Figures

Similar articles
-
Practice-level effects of interventions to improve asthma care in primary care settings: the Pediatric Asthma Care Patient Outcomes Research Team.Health Serv Res. 2005 Dec;40(6 Pt 1):1737-57. doi: 10.1111/j.1475-6773.2005.00451.x. Health Serv Res. 2005. PMID: 16336546 Free PMC article. Clinical Trial.
-
Adherence to Asthma Guidelines in Children, Tweens, and Adults in Primary Care Settings: A Practice-Based Network Assessment.Mayo Clin Proc. 2016 Apr;91(4):411-21. doi: 10.1016/j.mayocp.2016.01.010. Epub 2016 Mar 1. Mayo Clin Proc. 2016. PMID: 26944837 Free PMC article.
-
A multicenter observational study of US adults with acute asthma: who are the frequent users of the emergency department?J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):733-40. doi: 10.1016/j.jaip.2014.06.012. Epub 2014 Sep 10. J Allergy Clin Immunol Pract. 2014. PMID: 25439365
-
Comparative effectiveness of asthma interventions within a practice based research network.BMC Health Serv Res. 2011 Aug 16;11:188. doi: 10.1186/1472-6963-11-188. BMC Health Serv Res. 2011. PMID: 21846401 Free PMC article. Review.
-
Training Staff at Doctors' Offices to Use Shared Decision-Making with Patients Choosing Asthma Treatments [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jul. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jul. PMID: 39556675 Free Books & Documents. Review.
Cited by
-
Elevating the Importance of Asthma Care in the United States.Fed Pract. 2024 Nov;41(Suppl 6):S13-S22. doi: 10.12788/fp.0531. Epub 2024 Nov 20. Fed Pract. 2024. PMID: 39839065 Free PMC article. No abstract available.
-
Approaches to Management of Asthma: Guidelines for Stepped Care and Self-Monitoring.Adv Exp Med Biol. 2023;1426:355-375. doi: 10.1007/978-3-031-32259-4_15. Adv Exp Med Biol. 2023. PMID: 37464128
-
Difficult vs. Severe Asthma: Definition and Limits of Asthma Control in the Pediatric Population.Front Pediatr. 2018 Jun 19;6:170. doi: 10.3389/fped.2018.00170. eCollection 2018. Front Pediatr. 2018. PMID: 29971223 Free PMC article. Review.
-
Best practice advice for asthma exacerbation prevention and management in primary care: an international expert consensus.NPJ Prim Care Respir Med. 2024 Nov 17;34(1):39. doi: 10.1038/s41533-024-00399-2. NPJ Prim Care Respir Med. 2024. PMID: 39551807 Free PMC article. Review.
-
Is It Time for a Patient-Centered Quality Measure of Asthma Control?J Allergy Clin Immunol Pract. 2019 Jul-Aug;7(6):1771-1777. doi: 10.1016/j.jaip.2019.02.016. Epub 2019 Apr 4. J Allergy Clin Immunol Pract. 2019. PMID: 30954466 Free PMC article. Review.
References
-
- Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. Vital Health Stat 10. 2010; 247(247): 1–82. - PubMed
-
- Lozano P, Sullivan SD, Smith DH, Weiss KB. The economic burden of asthma in US children: estimates from the National Medical Expenditure Survey. J Allergy Clin Immunol. 1999; 104(5): 957–963. - PubMed
-
- To T, Dell S, Dick P, Cicutto L. The burden of illness experienced by young children associated with asthma: a population-based cohort study. J Asthma. 2008; 45(1): 45–49. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
