Potential Role for Regulatory B Cells as a Major Source of Interleukin-10 in Spleen from Plasmodium chabaudi-Infected Mice
- PMID: 29531131
- PMCID: PMC5913855
- DOI: 10.1128/IAI.00016-18
Potential Role for Regulatory B Cells as a Major Source of Interleukin-10 in Spleen from Plasmodium chabaudi-Infected Mice
Abstract
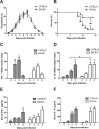
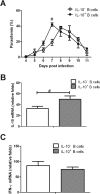
Interleukin-10 (IL-10)-producing regulatory B (Breg) cells were found to be induced in a variety of infectious diseases. However, its importance in the regulation of immune response to malaria is still unclear. Here, we investigated the dynamics, phenotype, and function of Breg cells using Plasmodium chabaudi chabaudi AS-infected C57BL/6 and BALB/c mice. BALB/c mice were more susceptible to infection and had a stronger IL-10 response in spleen than C57BL/6 mice. Analysis of the surface markers of IL-10-producing cells with flow cytometry showed that CD19+ B cells were one of the primary IL-10-producing populations in P. c. chabaudi AS-infected C57BL/6 and BALB/c mice, especially in the latter one. The Breg cells had a heterogeneous phenotype which shifted during infection. The well-established Breg subset, CD19+ CD5+ CD1dhi cells, accounted for less than 20% of IL-10-producing B cells in both strains during the course of infection. Most Breg cells were IgG+ and CD138- from day 0 to day 8 postinfection. Adoptive transfer of Breg cells to C57BL/6 mice infected with P. c. chabaudi AS led to a transient increase of parasitemia without an impact on survival rate. Our finding reveals that B cells play an active and important regulatory role in addition to mediating humoral immunity in immune response against malaria, which should be paid more attention in developing therapeutic or vaccine strategies against malaria involving stimulation of B cells.
Keywords: B10; IL-10; P. c. chabaudi AS; malaria; regulatory B cells.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Role of IL-10-producing regulatory B cells in control of cerebral malaria in Plasmodium berghei infected mice.Eur J Immunol. 2013 Nov;43(11):2907-18. doi: 10.1002/eji.201343512. Epub 2013 Aug 27. Eur J Immunol. 2013. PMID: 23893352
-
Expansion of IL-3-responsive IL-4-producing non-B non-T cells correlates with anemia and IL-3 production in mice infected with blood-stage Plasmodium chabaudi malaria.Eur J Immunol. 1998 Aug;28(8):2559-70. doi: 10.1002/(SICI)1521-4141(199808)28:08<2559::AID-IMMU2559>3.0.CO;2-M. Eur J Immunol. 1998. PMID: 9710233
-
IL-2 contributes to maintaining a balance between CD4+Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection.J Immunol. 2011 Apr 15;186(8):4862-71. doi: 10.4049/jimmunol.1003777. Epub 2011 Mar 9. J Immunol. 2011. PMID: 21389253
-
A dual role for B cells in Plasmodium chabaudi chabaudi (AS) infection?Res Immunol. 1994 Jul-Aug;145(6):412-9. doi: 10.1016/s0923-2494(94)80170-3. Res Immunol. 1994. PMID: 7899705 Review.
-
Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection.Immunol Rev. 2004 Oct;201:35-47. doi: 10.1111/j.0105-2896.2004.00182.x. Immunol Rev. 2004. PMID: 15361231 Review.
Cited by
-
Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host?Pathogens. 2021 Oct 3;10(10):1277. doi: 10.3390/pathogens10101277. Pathogens. 2021. PMID: 34684226 Free PMC article. Review.
-
Single cell transcriptomics shows that malaria promotes unique regulatory responses across multiple immune cell subsets.Nat Commun. 2023 Nov 15;14(1):7387. doi: 10.1038/s41467-023-43181-7. Nat Commun. 2023. PMID: 37968278 Free PMC article.
-
The impact of Plasmodium-driven immunoregulatory networks on immunity to malaria.Nat Rev Immunol. 2024 Sep;24(9):637-653. doi: 10.1038/s41577-024-01041-5. Epub 2024 Jun 11. Nat Rev Immunol. 2024. PMID: 38862638 Free PMC article. Review.
-
The interleukin-10 family: Major regulators of the immune response against Plasmodium falciparum infections.Saudi J Biol Sci. 2023 Nov;30(11):103805. doi: 10.1016/j.sjbs.2023.103805. Epub 2023 Sep 6. Saudi J Biol Sci. 2023. PMID: 37727525 Free PMC article. Review.
-
Immunomodulatory activity of extracts from five edible basidiomycetes mushrooms in Wistar albino rats.Sci Rep. 2022 Jul 20;12(1):12423. doi: 10.1038/s41598-022-16349-2. Sci Rep. 2022. PMID: 35859110 Free PMC article.
References
-
- World Health Organization. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

