Changes in Cerebral Arteries and Parenchymal Arterioles With Aging: Role of Rho Kinase 2 and Impact of Genetic Background
- PMID: 29531174
- PMCID: PMC5897142
- DOI: 10.1161/HYPERTENSIONAHA.118.10865
Changes in Cerebral Arteries and Parenchymal Arterioles With Aging: Role of Rho Kinase 2 and Impact of Genetic Background
Abstract
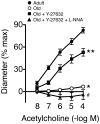
Vascular aging fundamentally contributes to large and small vessel disease. Despite the importance of such changes for brain function, mechanisms that mediate such changes are poorly defined. We explored mechanisms that underlie changes with age, testing the hypothesis that ROCK (Rho kinase) plays an important role. In C57BL/6 mice, baseline diameters of isolated pressurized parenchymal arterioles were similar in adult (4-5 month) and old mice (22±1 month; ≈15±1 µm). Endothelium-dependent dilation was impaired in old mice compared with adults in a pathway-specific manner. Vasodilation to NS-309 (which activates small- and intermediate-conductance Ca2+ activated K+ channels in endothelial cells) was intact while endothelial nitric oxide synthase-mediated vasodilation was reduced by ≥60%, depending on the concentration (P<0.05). A similar reduction was present in basilar arteries. Inhibiting both ROCK isoforms with Y-27632 restored the majority of endothelial function in old mice. Because genetic background is a determinant of vascular disease, we performed similar studies using FVB/N mice. Endothelial dysfunction was seen with aging in both FVB/N and C57BL/6 mice although the magnitude was increased ≈2-fold in the latter strain (P<0.05). In both strains of mice, age-induced endothelial dysfunction was reversed by inhibition of ROCK2 with SLX-2119. Thus, aging impairs endothelial function in both cerebral arteries and parenchymal arterioles, predominantly via effects on endothelial nitric oxide synthase-dependent regulation of vascular tone. The magnitude of these changes was influenced by genetic background and mediated by ROCK2.
Keywords: endothelium; genetic background; nitric oxide; rho-associated kinases; vascular diseases.
© 2018 American Heart Association, Inc.
Figures

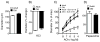


References
-
- Faraci F. Cerebral vascular dysfunction with aging. In: Masoro EJAS, editor. Handbook of the Biology of Aging. Academic Press; 2011. pp. 405–418.
-
- Nagata K, Yamazaki T, Takano D, Maeda T, Fujimaki Y, Nakase T, Sato Y. Cerebral circulation in aging. Ageing Res Rev. 2016;30:49–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

