Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS)
- PMID: 29531228
- PMCID: PMC5847601
- DOI: 10.1038/s41598-018-22438-y
Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS)
Abstract
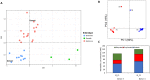
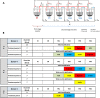
Consumption of fermentable dietary fibers (DFs), which can induce growth and/or activity of specific beneficial populations, is suggested a promising strategy to modulate the gut microbiota and restore health in microbiota-linked diseases. Until today, inulin and fructo-oligosaccharides (FOS) are the best studied DFs, while little is known about the gut microbiota-modulating effects of β-glucan, α-galactooligosaccharide (α-GOS) and xylo-oligosaccharide (XOS). Here, we used three continuous in vitro fermentation PolyFermS model to study the modulating effect of these DFs on two distinct human adult proximal colon microbiota, independently from the host. Supplementation of DFs, equivalent to a 9 g daily intake, induced a consistent metabolic response depending on the donor microbiota. Irrespective to the DF supplemented, the Bacteroidaceae-Ruminococcaceae dominated microbiota produced more butyrate (up to 96%), while the Prevotellaceae-Ruminococcaceae dominated microbiota produced more propionate (up to 40%). Changes in abundance of specific bacterial taxa upon DF supplementation explained the observed changes in short-chain fatty acid profiles. Our data suggest that the metabolic profile of SCFA profile may be the most suitable and robust read-out to characterize microbiota-modulating effects of a DF and highlights importance to understand the inter-individual response to a prebiotic treatment for mechanistic understanding and human application.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

