Shelf Life Evaluation of Clinical Grade Chondrogenic Induced Aged Adult Stem Cells for Cartilage Regeneration
- PMID: 29531282
- PMCID: PMC5847521
- DOI: 10.1038/s41598-018-22748-1
Shelf Life Evaluation of Clinical Grade Chondrogenic Induced Aged Adult Stem Cells for Cartilage Regeneration
Abstract
The study objectives include, enhancing the proliferations of aged bone marrow stem cells (BMSCs) and adipose stem cells (ADSCs); and evaluating the shelf lives of clinical grade chondrogenically induced cells from both samples. ADSCs and BMSCs from 56 patients (76 ± 8 yrs) were proliferated using basal medium (FD) and at (5, 10, 15, 20 and 25) ng/ml of basal fibroblast growth factor (bFGF). They were induced to chondrogenic lineage and stored for more than 120 hrs in FD, serum, Dulbecco's phosphate buffered saline (DPBS) and saline at 4 °C. In FD, cells stagnated and BMSCs' population doubling time (PDT) was 137 ± 30 hrs, while ADSCs' was 129.7 ± 40 hrs. bFGF caused PDT's decrease to 24.5 ± 5.8 hrs in BMSCs and 22.0 ± 6.5 hrs in ADSCs (p = 0.0001). Both cells were positive to stem cell markers before inductions and thereafter, expressed significantly high chondrogenic genes (p = 0.0001). On shelf life, both cells maintained viabilities and counts above 70% in FD and serum after 120 hrs. BMSCs' viabilities in DPBS fell below 70% after 96 hrs and saline after 72 hrs. ADSCs' viability fell below 70% in DPBS after 24 hrs and saline within 24 hrs. Concentrations between 20 ng/ml bFGF is ideal for aged adult cells' proliferation and delivery time of induced BMSCs and ADSCs can be 120 hrs in 4 °C serum.
Conflict of interest statement
The authors declare no competing interests.
Figures





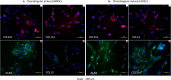
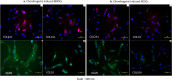
References
-
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint A prospective, comparative trial. The Journal of Bone and Joint Surgery (American). 2003;85:185–192. doi: 10.2106/00004623-200302000-00001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

