Sitagliptin Accelerates Endothelial Regeneration after Vascular Injury Independent from GLP1 Receptor Signaling
- PMID: 29531541
- PMCID: PMC5822806
- DOI: 10.1155/2018/5284963
Sitagliptin Accelerates Endothelial Regeneration after Vascular Injury Independent from GLP1 Receptor Signaling
Abstract
Introduction: DPP4 inhibitors (gliptins) are commonly used antidiabetic drugs for the treatment of type 2 diabetes. Gliptins also act in a glucose-independent manner and show vasoregenerative effects. We have shown that gliptins can remarkably accelerate vascular healing after vascular injury. However, the underlying mechanisms remain unclear. Here, we examined potential signaling pathways linking gliptins to enhanced endothelial regeneration.
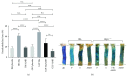
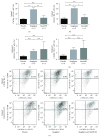
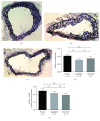
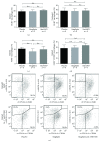
Methods and results: We used wild-type and GLP1 receptor knockout (Glp1r-/-) mice to investigate the underlying mechanisms of gliptin-induced reendothelialization. The prototype DPP4 inhibitor sitagliptin accelerated endothelial healing in both animal models. Improved endothelial growth was associated with gliptin-mediated progenitor cell recruitment into the diseased vascular wall via the SDF1-CXCR4 axis independent of GLP1R-dependent signaling pathways. Furthermore, SDF1 showed direct proproliferative effects on endothelial cells. Excessive neointimal formation was not observed in gliptin- or placebo-treated Glp1r-/- mice.
Conclusion: We identified the SDF1-CXCR4 axis as a crucial signaling pathway for endothelial regeneration after acute vascular injury. Furthermore, SDF1 can directly increase endothelial cell proliferation. Gliptin-mediated potentiation of endothelial regeneration was preserved in Glp1r-/- animals. Thus, gliptin-mediated endothelial regeneration proceeds through SDF-1/CXCR4 in a GLP1R-independent manner after acute vascular injury.
Figures


References
-
- Brenner C., Krankel N., Kuhlenthal S., et al. Short-term inhibition of DPP-4 enhances endothelial regeneration after acute arterial injury via enhanced recruitment of circulating progenitor cells. International Journal of Cardiology. 2014;177(1):266–275. doi: 10.1016/j.ijcard.2014.09.016. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

