Chemical signaling and insect attraction is a conserved trait in yeasts
- PMID: 29531709
- PMCID: PMC5838033
- DOI: 10.1002/ece3.3905
Chemical signaling and insect attraction is a conserved trait in yeasts
Abstract
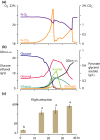
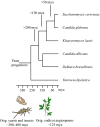
Yeast volatiles attract insects, which apparently is of mutual benefit, for both yeasts and insects. However, it is unknown whether biosynthesis of metabolites that attract insects is a basic and general trait, or if it is specific for yeasts that live in close association with insects. Our goal was to study chemical insect attractants produced by yeasts that span more than 250 million years of evolutionary history and vastly differ in their metabolism and lifestyle. We bioassayed attraction of the vinegar fly Drosophila melanogaster to odors of phylogenetically and ecologically distinct yeasts grown under controlled conditions. Baker's yeast Saccharomyces cerevisiae, the insect-associated species Candida californica, Pichia kluyveri and Metschnikowia andauensis, wine yeast Dekkera bruxellensis, milk yeast Kluyveromyces lactis, the vertebrate pathogens Candida albicans and Candida glabrata, and oleophilic Yarrowia lipolytica were screened for fly attraction in a wind tunnel. Yeast headspace was chemically analyzed, and co-occurrence of insect attractants in yeasts and flowering plants was investigated through a database search. In yeasts with known genomes, we investigated the occurrence of genes involved in the synthesis of key aroma compounds. Flies were attracted to all nine yeasts studied. The behavioral response to baker's yeast was independent of its growth stage. In addition to Drosophila, we tested the basal hexapod Folsomia candida (Collembola) in a Y-tube assay to the most ancient yeast, Y. lipolytica, which proved that early yeast signals also function on clades older than neopteran insects. Behavioral and chemical data and a search for selected genes of volatile metabolites underline that biosynthesis of chemical signals is found throughout the yeast clade and has been conserved during the evolution of yeast lifestyles. Literature and database reviews corroborate that yeast signals mediate mutualistic interactions between insects and yeasts. Moreover, volatiles emitted by yeasts are commonly found also in flowers and attract many insect species. The collective evidence suggests that the release of volatile signals by yeasts is a widespread and phylogenetically ancient trait, and that insect-yeast communication evolved prior to the emergence of flowering plants. Co-occurrence of the same attractant signals in yeast and flowers suggests that yeast-insect communication may have contributed to the evolution of insect-mediated pollination in flowers.
Keywords: Crabtree‐positive; chemical signaling; fermentation; floral volatiles; insect attraction; mimicry; olfaction; pollination.
Figures



References
-
- Albertazzi, E. , Cardillo, R. , Servi, S. , & Zucchi, G. (1994). Biogeneration of 2‐phenylethanol and 2‐phenylethylacetate important aroma components. Biotechnology Letters, 16, 491–496. https://doi.org/10.1007/BF01023331 - DOI
-
- Altschul, S. F. , Gish, W. , Miller, W. , Myers, E. W. , & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2 - DOI - PubMed
-
- Andreadis, S. S. , Witzgall, P. , & Becher, P. G. (2015). Survey of arthropod assemblages responding to live yeasts in an organic apple orchard. Frontiers in Ecology and Evolution, 3, 121.
-
- Arguello, J. R. , Sellanes, C. , Lou, Y. R. , & Raguso, R. A. (2013). Can yeast (S. cerevisiae) metabolic volatiles provide polymorphic signaling? PLoS ONE, 8, e70219 https://doi.org/10.1371/journal.pone.0070219 - DOI - PMC - PubMed
-
- Bailes, E. J. , Ollerton, J. , Pattrick, J. G. , & Glover, B. J. (2015). How can an understanding of plant‐pollinator interactions contribute to global food security? Current Opinion in Plant Biology, 26, 72–79. https://doi.org/10.1016/j.pbi.2015.06.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

