Ube2V2 Is a Rosetta Stone Bridging Redox and Ubiquitin Codes, Coordinating DNA Damage Responses
- PMID: 29532025
- PMCID: PMC5833000
- DOI: 10.1021/acscentsci.7b00556
Ube2V2 Is a Rosetta Stone Bridging Redox and Ubiquitin Codes, Coordinating DNA Damage Responses
Abstract
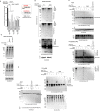
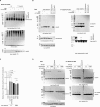
Posttranslational modifications (PTMs) are the lingua franca of cellular communication. Most PTMs are enzyme-orchestrated. However, the reemergence of electrophilic drugs has ushered mining of unconventional/non-enzyme-catalyzed electrophile-signaling pathways. Despite the latest impetus toward harnessing kinetically and functionally privileged cysteines for electrophilic drug design, identifying these sensors remains challenging. Herein, we designed "G-REX"-a technique that allows controlled release of reactive electrophiles in vivo. Mitigating toxicity/off-target effects associated with uncontrolled bolus exposure, G-REX tagged first-responding innate cysteines that bind electrophiles under true kcat/Km conditions. G-REX identified two allosteric ubiquitin-conjugating proteins-Ube2V1/Ube2V2-sharing a novel privileged-sensor-cysteine. This non-enzyme-catalyzed-PTM triggered responses specific to each protein. Thus, G-REX is an unbiased method to identify novel functional cysteines. Contrasting conventional active-site/off-active-site cysteine-modifications that regulate target activity, modification of Ube2V2 allosterically hyperactivated its enzymatically active binding-partner Ube2N, promoting K63-linked client ubiquitination and stimulating H2AX-dependent DNA damage response. This work establishes Ube2V2 as a Rosetta-stone bridging redox and ubiquitin codes to guard genome integrity.
Conflict of interest statement
The authors declare the following competing financial interest(s): Provisional patent for the G-REX method in filing stages (by Cornell University).
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

