Mechanisms of KCNQ1 channel dysfunction in long QT syndrome involving voltage sensor domain mutations
- PMID: 29532034
- PMCID: PMC5842040
- DOI: 10.1126/sciadv.aar2631
Mechanisms of KCNQ1 channel dysfunction in long QT syndrome involving voltage sensor domain mutations
Abstract
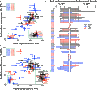
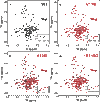
Mutations that induce loss of function (LOF) or dysfunction of the human KCNQ1 channel are responsible for susceptibility to a life-threatening heart rhythm disorder, the congenital long QT syndrome (LQTS). Hundreds of KCNQ1 mutations have been identified, but the molecular mechanisms responsible for impaired function are poorly understood. We investigated the impact of 51 KCNQ1 variants with mutations located within the voltage sensor domain (VSD), with an emphasis on elucidating effects on cell surface expression, protein folding, and structure. For each variant, the efficiency of trafficking to the plasma membrane, the impact of proteasome inhibition, and protein stability were assayed. The results of these experiments combined with channel functional data provided the basis for classifying each mutation into one of six mechanistic categories, highlighting heterogeneity in the mechanisms resulting in channel dysfunction or LOF. More than half of the KCNQ1 LOF mutations examined were seen to destabilize the structure of the VSD, generally accompanied by mistrafficking and degradation by the proteasome, an observation that underscores the growing appreciation that mutation-induced destabilization of membrane proteins may be a common human disease mechanism. Finally, we observed that five of the folding-defective LQTS mutant sites are located in the VSD S0 helix, where they interact with a number of other LOF mutation sites in other segments of the VSD. These observations reveal a critical role for the S0 helix as a central scaffold to help organize and stabilize the KCNQ1 VSD and, most likely, the corresponding domain of many other ion channels.
Figures



References
-
- Jespersen T., Grunnet M., Olesen S.-P., The KCNQ1 potassium channel: From gene to physiological function. Physiology 20, 408–416 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

