Association of Progressive Multifocal Leukoencephalopathy Lesion Volume With JC Virus Polymerase Chain Reaction Results in Cerebrospinal Fluid of Natalizumab-Treated Patients With Multiple Sclerosis
- PMID: 29532061
- PMCID: PMC5885213
- DOI: 10.1001/jamaneurol.2018.0094
Association of Progressive Multifocal Leukoencephalopathy Lesion Volume With JC Virus Polymerase Chain Reaction Results in Cerebrospinal Fluid of Natalizumab-Treated Patients With Multiple Sclerosis
Abstract
Importance: The JC virus (JCV) was named after the first patient to be described with progressive multifocal leukoencephalopathy (PML), John Cunningham. Detection of JC virus DNA in cerebrospinal fluid (CSF) by polymerase chain reaction (PCR), and of specific lesions by brain magnetic resonance imaging (MRI), are both considered essential for the diagnosis of natalizumab-associated PML (NTZ-PML) in patients with multiple sclerosis. However, strict pharmacovigilance by MRI can result in detection of patients with small lesions and undetectable JCV DNA in CSF.
Objective: To investigate the association of PML lesion characteristics on MRI with both qualitative and quantitative JCV PCR results in CSF of patients with NTZ-PML.
Design, setting and participants: This was a retrospective, cross-sectional study conducted from January 2007 to December 2014 in patients considered to have NTZ-PML based on a set of predefined criteria. Follow-up was at least 6 months. Data of patients from the Dutch-Belgian NTZ-PML cohort and patients treated at multiple medical centers in Belgium and the Netherlands and selected for research purposes were included as a convenience sample.
Main outcomes and measures: Brain MRI scans were analyzed for PML lesion volume, location, dissemination, and signs of inflammation. Associations of the qualitative and quantitative CSF JCV PCR results with PML MRI characteristics were calculated.
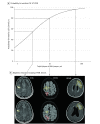
Results: Of the 73 patients screened, 56 were included (37 were women). At inclusion, 9 patients (16.1%) had undetectable JCV DNA in CSF. Patients with a positive PCR had larger total PML lesion volumes than those with undetectable JCV DNA (median volume, 22.9 mL; interquartile range, 9.2-60.4 mL vs median volume, 6.7 mL; interquartile range, 4.9-14.7 mL; P = .008), and logistic regression showed that a lower PML lesion volume significantly increased the probability for undetectable JCV DNA. There was a positive correlation between PML lesion volume and JCV copy numbers (Spearman ρ, 0.32; P = .03). Progressive multifocal leukoencephalopathy lesion volume was higher in patients with PML symptoms and in patients with more widespread lesion dissemination. No association was found between PCR results and PML lesion dissemination, signs of inflammation, or PML symptoms.
Conclusions and relevance: Smaller NTZ-PML lesions are associated with a higher likelihood of undetectable JCV DNA in CSF. This may preclude a formal diagnosis of PML and can complicate patient treatment in patients with small MRI lesions highly suggestive of PML detected early through pharmacovigilance.
Conflict of interest statement
Figures
Comment in
-
Progressive Multifocal Leukoencephalopathy Lesions and JC Virus: The Limits and Value of Imaging.JAMA Neurol. 2018 Jul 1;75(7):789-790. doi: 10.1001/jamaneurol.2018.0004. JAMA Neurol. 2018. PMID: 29532060 No abstract available.
Similar articles
-
Performance of PML diagnostic criteria in natalizumab-associated PML: data from the Dutch-Belgian cohort.J Neurol Neurosurg Psychiatry. 2019 Jan;90(1):44-46. doi: 10.1136/jnnp-2018-318261. Epub 2018 Aug 12. J Neurol Neurosurg Psychiatry. 2019. PMID: 30100552
-
Atypical Multiple Sclerosis Lesions or Progressive Multifocal Leukoencephalopathy Lesions: That Is the Question.J Investig Med High Impact Case Rep. 2020 Jan-Dec;8:2324709620939802. doi: 10.1177/2324709620939802. J Investig Med High Impact Case Rep. 2020. PMID: 32646245 Free PMC article.
-
Natalizumab-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: Findings from an Italian Independent Registry.PLoS One. 2016 Dec 20;11(12):e0168376. doi: 10.1371/journal.pone.0168376. eCollection 2016. PLoS One. 2016. PMID: 27997580 Free PMC article. Clinical Trial.
-
Anti-JCV serology during natalizumab treatment: Review and meta-analysis of 17 independent patient cohorts analyzing anti-John Cunningham polyoma virus sero-conversion rates under natalizumab treatment and differences between technical and biological sero-converters.Mult Scler. 2018 Apr;24(5):563-573. doi: 10.1177/1352458517728814. Epub 2017 Aug 29. Mult Scler. 2018. PMID: 28847222 Review.
-
[Idiopathic CD4-positive lymphocytopenia-associated progressive multifocal leukoencephalopathy confirmed by brain biopsy following negative results of repeated CSF-JC-virus tests: a case report].Rinsho Shinkeigaku. 2018 Dec 21;58(12):750-755. doi: 10.5692/clinicalneurol.cn-001227. Epub 2018 Nov 29. Rinsho Shinkeigaku. 2018. PMID: 30487366 Review. Japanese.
Cited by
-
Progressive Multifocal Leukoencephalopathy in a Patient with Multifocal Neurological Manifestations Caused by Solitary Brainstem Involvement.Intern Med. 2023 Mar 1;62(5):787-792. doi: 10.2169/internalmedicine.9627-22. Epub 2022 Aug 10. Intern Med. 2023. PMID: 35945023 Free PMC article.
-
Performance of Ultrasensitive Polymerase Chain Reaction Testing for JC Polyomavirus in Cerebrospinal Fluid Compared with Pathological Diagnosis of Progressive Multifocal Leukoencephalopathy.Viruses. 2024 Dec 19;16(12):1950. doi: 10.3390/v16121950. Viruses. 2024. PMID: 39772255 Free PMC article.
-
An atypical case of progressive multifocal leukoencephalopathy in a patient with high grade B-cell lymphoma causing diagnostic delay.JRSM Open. 2023 Oct 9;14(10):20542704231200395. doi: 10.1177/20542704231200395. eCollection 2023 Oct. JRSM Open. 2023. PMID: 37822464 Free PMC article.
-
Development of demyelinating lesions in progressive multifocal leukoencephalopathy (PML): Comparison of magnetic resonance images and neuropathology of post-mortem brain.Neuropathology. 2019 Aug;39(4):294-306. doi: 10.1111/neup.12562. Epub 2019 Jun 2. Neuropathology. 2019. PMID: 31155757 Free PMC article.
-
Ocrelizumab for the Treatment of Multiple Sclerosis: Safety, Efficacy, and Pharmacology.Ther Clin Risk Manag. 2021 Jul 30;17:765-776. doi: 10.2147/TCRM.S282390. eCollection 2021. Ther Clin Risk Manag. 2021. PMID: 34354358 Free PMC article. Review.
References
-
- Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438-446. - PubMed
-
- Rudick R, Polman C, Clifford D, Miller D, Steinman L. Natalizumab: bench to bedside and beyond. JAMA Neurol. 2013;70(2):172-182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

